Introduction
In the dynamic landscape of clinical research, efficient data management is paramount to advancing medical knowledge and improving patient outcomes. The article delves into the multifaceted approaches to optimizing clinical data management, starting with identifying inefficiencies in current workflows. It emphasizes the need for comprehensive workflow assessments and the strategic integration of data management plans early in the trial process.
Further, it explores the transformative role of technology, outlining how electronic data capture systems and mobile technologies enhance data accuracy and streamline regulatory submissions.
The discussion extends to the automation of clinical data management processes, highlighting how automation reduces administrative burdens and ensures data integrity. It also examines the implementation of centralized data management systems, which offer unified platforms for data collection, storage, and analysis, crucial for handling the complexity and volume of modern clinical trials. The article underscores the importance of rigorous data quality control measures and the adoption of advanced electronic data collection systems to ensure reliable study outcomes.
Finally, the integration of diverse data management systems for seamless data exchange is addressed, focusing on the role of application programming interfaces (APIs) in facilitating effective communication between systems. This comprehensive overview provides valuable insights into the strategies and technologies that can significantly enhance the efficiency and accuracy of clinical data management, ultimately driving the success of clinical trials.
Identifying Inefficiencies in Current Workflows
Efficient clinical information oversight relies on a thorough grasp of current processes. Inefficiencies often manifest as entry redundancies, extended validation timelines, and communication gaps among team members. Conducting a detailed assessment of current practices is essential to uncover bottlenecks and typical delay points. Employing process mapping techniques can visually represent workflows, highlighting inefficiencies and enabling prioritization of improvements.
A study on documentation burden found that 62.5% of 48 analyzed studies were performed in single institutions, highlighting a common challenge of fragmented information management. This reflects the broader issue within clinical trials, where information collection infrastructure remains labor-intensive and expensive. There is a pressing need to integrate various information sources and streamline digital flows to enhance efficiency.
Workshops and hybrid meetings can facilitate knowledge sharing and governance, as demonstrated by a project team that held face-to-face and online events to accommodate all team members. This inclusive approach ensures that even clinicians with demanding schedules can participate, fostering a unified strategy.
'Sabrina Steffen, Head of Information Strategy and Innovation for Information Handling at IQVIA, emphasizes the importance of incorporating an information handling approach early in trial planning.'. By doing so, teams can better manage the influx of information from various sources, which has increased threefold over the past decade. Typically, a research trial currently processes around 8.1 million information points, highlighting the need for an effective information handling system.
In conclusion, tackling inefficiencies in healthcare information oversight necessitates a multifaceted strategy, involving comprehensive workflow evaluations, the application of process mapping, and early incorporation of information strategies. This holistic method can significantly enhance trials, ultimately resulting in better patient outcomes.
The Role of Technology in Enhancing Efficiency
Technology is transforming clinical information management by introducing efficiency and accuracy into the process. The use of electronic information collection (EDC) tools, including MedTech-focused platforms like Greenlight Guru Clinical, significantly decreases the time and mistakes related to manual information entry. These systems are designed with user-friendly interfaces, facilitating quick onboarding without the need for technical expertise.
Moreover, mobile technologies enable real-time information collection at the site level, ensuring timely updates and reducing delays. 'This is essential for preserving the integrity and adherence of research trials, as mismanagement of patient information can lead to considerable inefficiencies and risks, particularly in high-cost areas like cell and gene therapy studies.'.
The significance of outstanding healthcare information organization cannot be exaggerated. Thorough information gathering is the backbone of submissions to regulatory bodies, ensuring that medical devices are safe and effective for market approval. For example, around 10-15% of effective 510(k) applications for Class II devices in the US depend on trial information, highlighting the importance of strong information management frameworks.
By utilizing modern technology, organizations can improve information accuracy, streamline the pathway to regulatory submissions, and ultimately generate more revenue. The shift from traditional paper-based case report forms (CRFs) to electronic CRFs (eCRFs) has shown to increase efficiency and reduce errors, making it a preferred choice for many medical device companies. Therefore, incorporating sophisticated EDC systems and mobile technologies in research trials not only guarantees adherence and information integrity but also speeds up the decision-making process, promoting innovation and enhancing patient results.
Automating Clinical Data Management Processes
Automation is transforming clinical information management by vastly enhancing efficiency and accuracy. By automating routine tasks such as information entry, validation, and reporting, organizations can significantly reduce the time researchers spend on administrative work, allowing them to focus more on critical analysis and interpretation. Electronic Information Gathering (EIG) tools, for example, streamline the gathering and storage of patient information in a digital format. These frameworks incorporate integrated validation measures, which improve information reliability and guarantee adherence to regulatory standards.
Within a vast unified health service framework in the United States, automation has played a key role in converting unorganized medical information into practical insights. Tools like automated information monitoring systems can identify discrepancies in real-time, enabling immediate resolution and ensuring integrity. 'The Department of Veterans Affairs' Health Information and Analytics Platform (HDAP) exemplifies this approach, leveraging a scalable, cloud-native enterprise analytics platform to support large-scale projects and medical decision-making.
Furthermore, automation technologies include built-in checks and reminders, which enhance patient adherence to reporting requirements. This results in improved information quality and decreases the chances of errors, contributing to more dependable and compliant trial outcomes. As Dan Herron, VP of digital offerings at RWS Life Sciences, observes, 'In large-scale research trials involving thousands of patients, manual information gathering is time-consuming and prone to human error.'. EDC systems are a game-changer, automating data collection and enhancing data reliability.”
The integration of automation in clinical trials is further bolstered by AI advancements. For example, John Snow Labs' generative AI models have been pivotal in optimizing healthcare workflows, and Qure.ai’s partnership with Project Data Sphere aims to enhance tumor assessments using AI-enabled solutions. These innovations not only enhance the efficiency and reliability of trials but also improve patient outcomes by offering prompt and precise information for decision-making.
Implementing Centralized Data Management Systems
Centralized information management platforms provide a cohesive environment for gathering, storing, and analyzing, ensuring uniformity and precision across datasets. 'This approach is crucial given the complexity and volume of information in modern clinical trials, where studies can integrate up to 20 different sources, capturing billions of points.'. By merging information from diverse sources into a unified platform, organizations can improve cooperation and speed up the information review process, making details more available to all parties engaged in the study.
The execution of such frameworks has demonstrated remarkable success in diverse settings. For instance, the Department of Veterans Affairs (VA) developed the Health Information and Analytics Platform (HDAP), a scalable, cloud-native enterprise analytics platform aimed at enabling big projects. This platform builds on the VA's extensive history of electronic health record (EHR) innovation, which includes a Corporate Data Warehouse registered with over 24 million patients. Comparable models have been embraced by other healthcare organizations to enhance medical decision-making, population health oversight, and quality assessment.
Additionally, centralized information management frameworks assist the updated infrastructure required for medical research. Conventional techniques for gathering information in restricted environments are labor-intensive and frequently do not succeed in obtaining thorough participant details, including social factors affecting health. In comparison, centralized systems aid in the integration and harmonization of practice information with trials, surmounting these restrictions and improving the validity and generalizability of research results.
The Cancer and COVID-19 Consortium (CCC19) exemplifies an effective information management strategy. 'CCC19's framework for optimizing information gathering seeks to connect the divide between research results and practical uses. 'Their quality control approach, inspired by the Center for International Blood and Marrow Transplant Research (CIBMTR), employs a quality score framework to objectively enhance information accuracy and completeness, even permitting the reduction of unknown and missing information without compromising respondent anonymity.'.
As medical testing progresses, the necessity for strong, centralized information management solutions becomes more evident. These systems not only ensure adherence to Good Clinical Practice (GCP) and other regulatory standards but also enable a more efficient and accurate information flow, ultimately driving advancements in medical knowledge and improving patient outcomes.
Best Practices for Clinical Data Quality and Control
Ensuring high information quality is paramount for reliable clinical study outcomes. Implementing rigorous information quality control measures, including regular audits and validation checks, significantly enhances reliability. Training personnel on optimal methods for information entry and monitoring can minimize mistakes, while creating clear governance policies guarantees accountability and adherence to regulatory standards.
In the area of medical research, the quality of information produced is vital for regulatory approvals. As per Article 62 of the European Union Medical Device Regulation (EU MDR), trial information must be scientifically valid, dependable, and strong. This is not only essential for protecting the rights and well-being of study participants but also for demonstrating the safety and efficacy of medical devices. In the U.S., roughly 10-15% of successful 510(k) submissions for Class II devices are founded on information from clinical trials, and all Class III devices necessitate extensive clinical trials to determine their safety and effectiveness.
Contemporary electronic information gathering (EDC) platforms provide sophisticated features that improve information quality. Pre-validated fields in EDC systems can alert investigators when entries fall outside acceptable ranges, thereby catching errors before they are recorded. This is particularly valuable in reducing mistakes due to fatigue or oversight. The adoption of electronic case report forms (eCRFs) has increased due to their higher efficiency and lower error rates compared to traditional paper-based CRFs.
The U.S. Food and Drug Administration (FDA) has highlighted the significance of trustworthy information in premarket submissions. The agency has noticed a rise in untrustworthy information from third-party labs, especially from organizations in China and India. The FDA's vigilance emphasizes the necessity for companies to guarantee the quality of information gathered on their behalf, as unreliable information can result in delays or rejections in regulatory approvals.
To tackle the shortcomings of conventional information gathering techniques, there is a movement towards updating the information framework for medical research. This includes integrating real-world information sources such as electronic health records (EHRs) and leveraging advanced collection tools. By doing so, researchers can capture a more comprehensive and accurate picture of patient outcomes, which is crucial for both regulatory submissions and the advancement of medical knowledge.
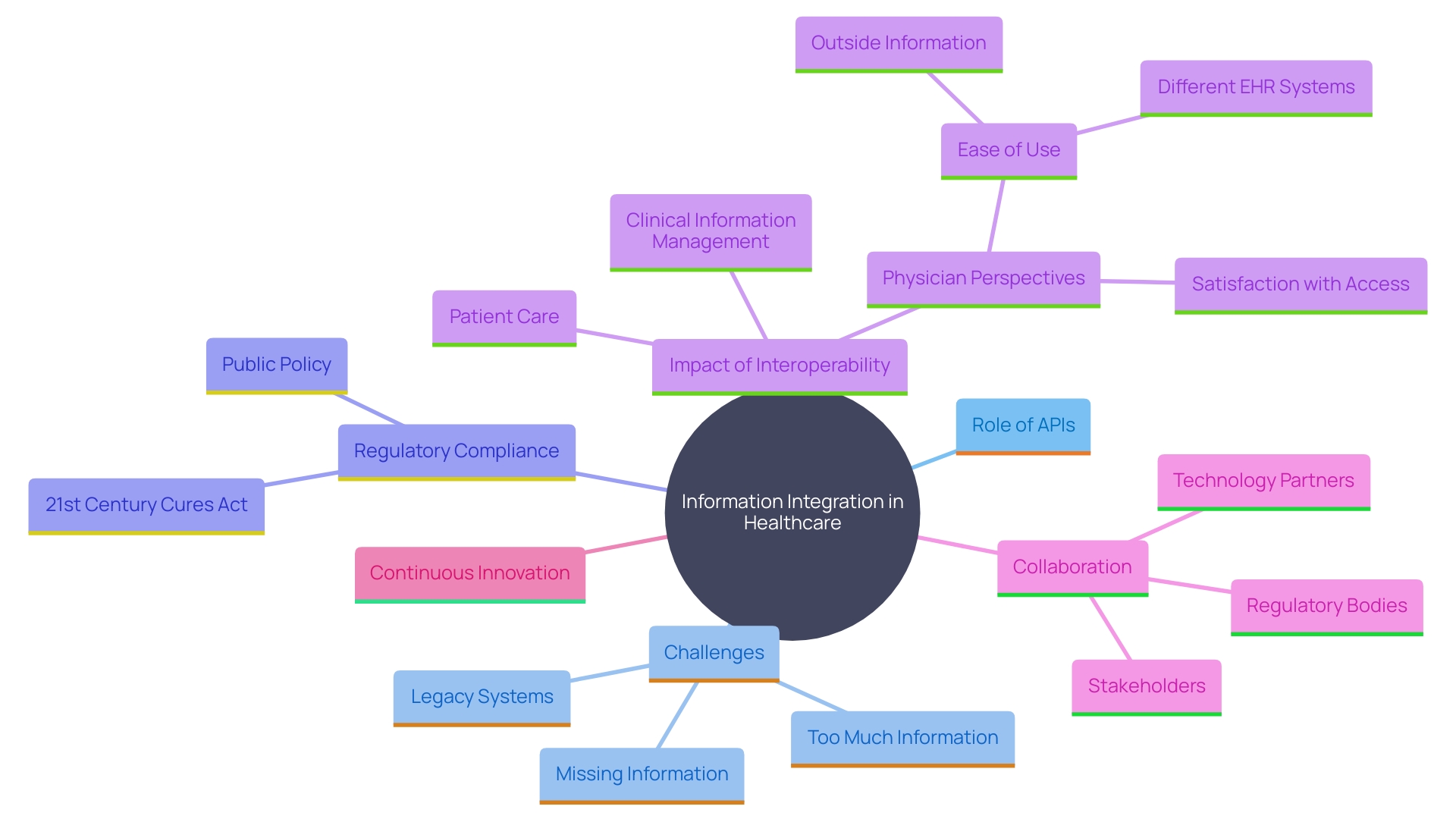
Integrating Systems for Seamless Data Exchange
The combination of varied information handling frameworks is essential for smooth information transfer in medical research. Utilizing application programming interfaces (APIs) enables effective communication between these systems, ensuring a smooth transfer of information across platforms. This integration not only minimizes the risk of information loss but also significantly enhances the efficiency of clinical information management by streamlining workflows and providing quicker access to vital details.
For instance, the Department of Veterans Affairs (VA) has made notable strides with its Health Information and Analytics Platform (HDAP), a scalable, cloud-native enterprise analytics platform aimed at enabling big projects. This initiative highlights the significance of strong infrastructure in facilitating efficient integration processes. The VA's Corporate Data Warehouse, which has recorded over 24 million patients, demonstrates the capability of such structures to manage large quantities of intricate healthcare information efficiently.
However, the healthcare sector faces significant challenges in achieving interoperability due to the prevalence of legacy systems and the lack of standardized information formats. Addressing these challenges requires deploying advanced integration technologies, such as middleware solutions and standardized APIs. As per a Deloitte survey, 47% of healthcare organizations mention regulatory compliance as a significant obstacle to information integration. 'Following stringent privacy rules such as HIPAA and GDPR is vital, requiring strong protection strategies, including advanced encryption and secure information transfer protocols.'.
In a recent survey, the overall engagement in interoperable exchange among hospitals increased from 46% to 70% between 2018 and 2023. Regularly interoperable hospitals indicated that 92% had the required health information accessible from external providers at the point of care, in contrast to just 33% of non-interoperable hospitals. This highlights the critical role of standardization and interoperability in improving patient care continuity and reducing information gaps.
The growing adoption of APIs in healthcare, driven by federal initiatives such as the 2015 Edition Cures Update, further emphasizes the need for standardized solutions. As healthcare systems continue to evolve, integrating modernized infrastructure and leveraging advanced data management solutions will be pivotal in transforming clinical research and enhancing patient outcomes.
Conclusion
Efficient clinical data management is essential for advancing medical research and improving patient care. Identifying inefficiencies in current workflows through comprehensive assessments and process mapping can significantly enhance the clinical trial process. By addressing these bottlenecks, organizations can streamline operations and promote better collaboration among teams, ultimately leading to improved patient outcomes.
The transformative role of technology cannot be overstated. The implementation of electronic data capture systems and mobile technologies has revolutionized data collection, reducing errors and expediting regulatory submissions. Automation further enhances these processes, allowing researchers to focus on critical analysis rather than administrative tasks.
As organizations embrace automation and advanced technologies, the reliability and speed of clinical data management are markedly improved.
Centralized data management systems provide a unified platform for data collection and analysis, essential for navigating the complexity of modern clinical trials. These systems not only enhance data integrity but also facilitate integration across various data sources, ensuring that all stakeholders have access to accurate information. The adoption of rigorous data quality control measures further reinforces the reliability of clinical study outcomes, which is vital for regulatory approvals.
Finally, the integration of diverse data management systems through application programming interfaces (APIs) ensures seamless data exchange, addressing the challenges of interoperability within the healthcare sector. By overcoming these barriers, organizations can enhance the continuity of care and ultimately drive advancements in medical knowledge. Collectively, these strategies underscore the importance of robust clinical data management in the pursuit of effective and efficient clinical trials.




