Introduction
Contract Research Organizations (CROs) play a pivotal role in streamlining clinical trials and enhancing the efficiency of medical research. With their comprehensive support and meticulous planning, CROs ensure that every aspect of the clinical trial process is optimized.
From managing patient recruitment to data administration and adherence to regulatory standards, CROs enable researchers to focus on essential tasks, accelerating the progression of research and facilitating medical breakthroughs. This article explores the benefits of CRO services in medical research, highlights successful partnerships in California, addresses challenges in rare disease clinical trials, and discusses the future of medical research with CRO services.
Benefits of CRO Services in Medical Research
Contract Research Organizations (CROs) are pivotal in streamlining the complex processes of clinical trials, particularly when trials span international boundaries. Take, for example, a patient from rural Pennsylvania with an ultra-rare disease and no approved treatment options. They're presented with the chance to join a clinical trial in Turkey, an opportunity that could be lifesaving.
Yet, the excitement is tempered by daunting logistical challenges: navigating visa processes, handling unfamiliar paperwork, and coordinating travel in a foreign language. These considerations underscore the need for CROss to provide comprehensive support. As articulated by industry experts, the involvement of CROss isn't merely about conducting phases of a study; it's about meticulously planning and optimizing each decision well in advance.
Insights from transaction advisories reveal that a significant portion of clinical trial decisions, up to 80% based on 1,200 data points analyzed, could be improved with more diligent preparation and investment of time and resources by CROss. This strategic approach ensures that every link in the clinical trial chain is fortified, aligning with the optimal timeline and needs of the company. Thus, by collaborating with CROss, the intricacies of clinical trials are managed more effectively, enhancing the quality of medical research conducted in regions like California and beyond.
Enhancing Research Efficiency with CRO Services
Clinical Research Organizations (CROs) are pivotal in enhancing the efficiency of medical research. They possess specialized expertise in orchestrating clinical trials, which empowers them to refine procedures and maximize the allocation of resources. By adeptly managing patient recruitment, data administration, and adherence to regulatory standards, CROss facilitate a sharpened focus for researchers on pivotal tasks.
This concentration on essential duties propels the momentum of research, catalyzing a swifter progression to results and medical breakthroughs. The integration of Artificial Intelligence (AI) and Machine Learning (ML) leverages digital workflows and data storage, propelling the personalization of patient treatments and augmenting the efficiency and velocity of clinical trials. With the burgeoning volume of health data, the vision of a self-regulating clinical trial looms on the horizon, signaling a transformative leap in pharmacological proficiency, organizational efficacy, and patient outcomes.
The journey to such technological advancements begins with a well-crafted research question, laying the groundwork for controlled medical studies. These questions dictate the manipulation of variables and measurement of outcomes, with the imperative selection of an appropriate control group to ensure the validity of conclusions. The meticulous planning of experimental design is crucial to mitigate biases and confounding factors, ensuring the integrity of the research process.
Case Studies of Successful CRO Partnerships in California
Clinical trial companies and Contract Research Organizations (CROs) have been integral in elevating medical research, particularly through their strategic collaborations in California. A standout case in point is the alliance between a major pharmaceutical entity and a CRO, which culminated in the triumphant execution of an extensive clinical trial aimed at exploring a novel cancer therapy. This venture underscored the CRO's proficiency in trial management and its pivotal contribution to the research's conclusive success.
These instances are a testament to the critical role that CRO services play in propelling medical research. Notably, initiatives like Walgreens' commitment to harnessing their established community presence offer promising inroads to addressing healthcare disparities by integrating clinical research services within their extensive pharmacy network. Such efforts are poised to dismantle barriers to trial access, ensuring broader participation and advancing healthcare outcomes.
Challenges and Opportunities in CRO Services for Rare Disease Clinical Trials
Clinical Research Organizations (CROs) are at the forefront of addressing the intricate challenges associated with rare disease clinical trials. These trials are particularly demanding due to the low prevalence of each condition among the over 6,000 recognized rare diseases globally.
With as much as 10 percent of the world's population affected by rare conditions, the small number of patients for each disease complicates the recruitment process, diagnosis, and comprehension of the patient journey. This, in turn, affects the ability to establish a new therapy's safety, effectiveness, and cost-effectiveness.
CROs, however, are equipped with specialized networks and knowledge to navigate these obstacles. They employ strategic approaches to patient recruitment by utilizing patient registries, forging partnerships with patient advocacy groups, and implementing cutting-edge recruitment tactics, ensuring trials are adequately populated.
The collaboration extends across a spectrum of stakeholders including researchers, advocacy groups, federal agencies like the FDA, policymakers, and the patients themselves, all of whom have vested interests in the advancements of treatments for rare diseases. The Innovation and Value Initiative's white paper, titled "Valuing Rare Disease Treatments in Healthcare: Real Experience, Real Impact," highlights the complexities in assessing the value of treatments for rare diseases, such as the consensus on outcomes to be assessed and the scarcity of real-world data due to the small patient populations. The paper emphasizes the significance of understanding the patient journey and the progression of the disease over time when determining outcomes that matter to patients with rare diseases. By successfully overcoming these recruitment and data challenges, CROs not only facilitate the development of new therapies but also contribute to a broader understanding of rare diseases, which can, in turn, shed light on more common conditions like cancer and Parkinson's disease.
The Future of Medical Research in California with CRO Services
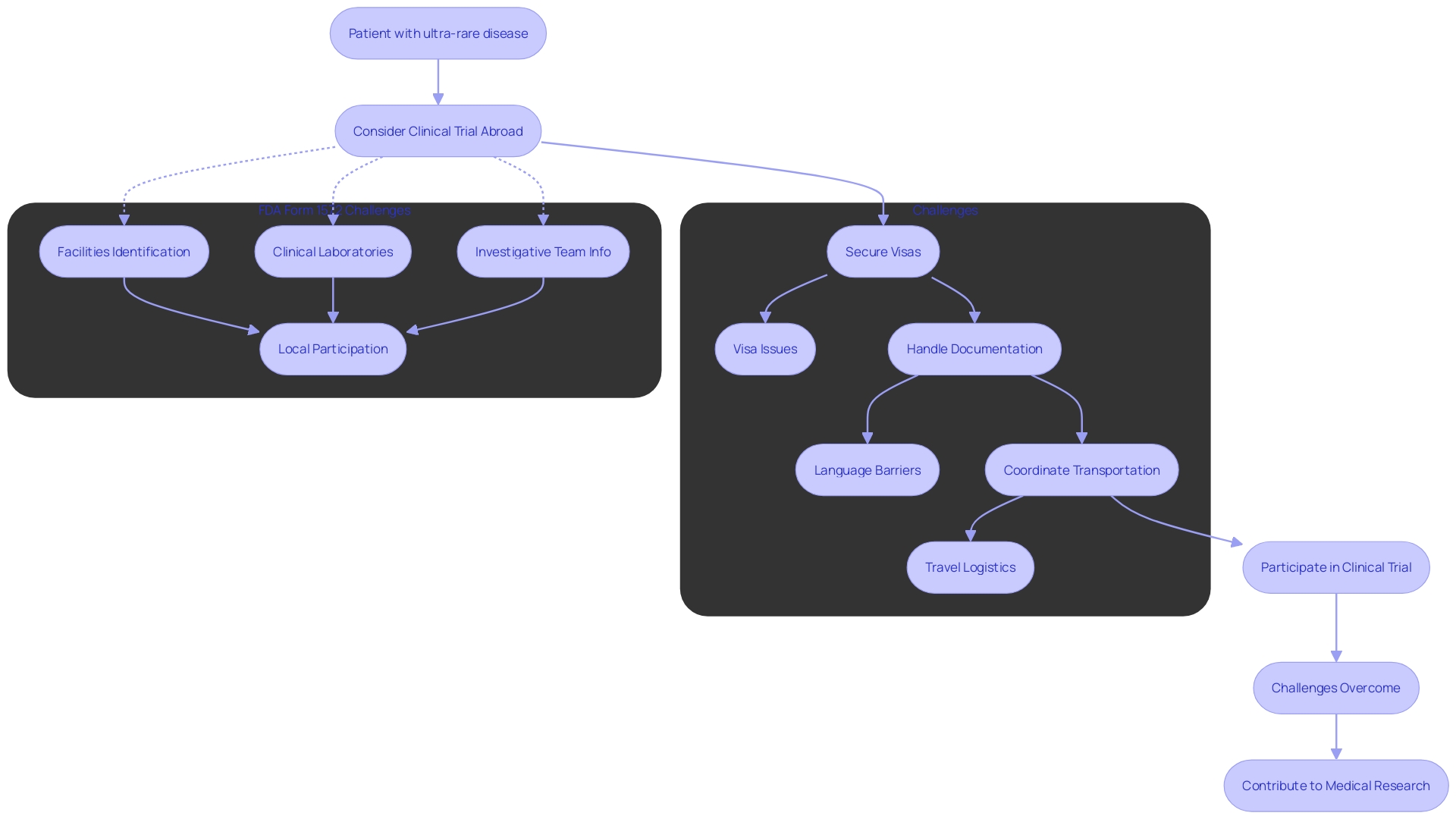
Clinical Research Organizations (CROs) are increasingly becoming the linchpin for managing the complexities of modern clinical trials, particularly as they expand in scope and geographical reach. Take, for example, the case of a patient from rural Pennsylvania who is battling an ultra-rare disease without any FDA-approved treatments. Presented with a chance to join a clinical trial in Turkey, the patient faces the daunting challenge of navigating international travel logistics.
From securing visas to handling unfamiliar documentation and coordinating various modes of transportation, the process is fraught with difficulties. CROs are equipped to address these challenges, providing essential support in patient recruitment and ensuring that participants can overcome the barriers to access groundbreaking medical research. Their role is vital not only in assisting individual patients but also in advancing global medical research endeavors.
Conclusion
In conclusion, Contract Research Organizations (CROs) play a pivotal role in streamlining clinical trials and enhancing the efficiency of medical research. By providing comprehensive support and meticulous planning, CROs optimize every aspect of the trial process, from patient recruitment to data administration and regulatory compliance.
CROs enhance research efficiency by refining procedures, maximizing resource allocation, and leveraging technologies like AI and ML. Successful partnerships between clinical trial companies and CROs in California highlight the critical contribution of CRO services to advancing medical research.
Challenges in rare disease trials are effectively addressed by CROs through specialized networks and strategic patient recruitment approaches. Overcoming these challenges not only facilitates the development of new therapies but also deepens our understanding of rare diseases.
Looking ahead, CROs will continue to play a vital role in managing complex trials as they expand globally. By assisting patients in accessing groundbreaking research, CROs contribute to advancing medical knowledge on a global scale. In summary, CRO services offer invaluable support in optimizing clinical trial processes, enhancing research efficiency, addressing challenges in rare disease trials, and shaping the future of medical research. With their comprehensive approach and strategic expertise, CROs drive advancements in healthcare and pave the way for medical breakthroughs.




