Introduction
Contract Research Organizations (CROs) play a vital role in advancing medical research. With their expertise in clinical trials, data management, and regulatory compliance, CROs offer researchers access to specialized skills and state-of-the-art facilities.
This collaboration can significantly enhance the efficiency and success rate of clinical endeavors while addressing complex ethical, legal, and social challenges. As the medical community continues to tackle global diseases, the strategic partnership with CROs becomes increasingly essential in improving patient outcomes and advancing medical science. In this article, we will explore the importance of CRO services in medical research, the challenges faced, successful case studies, the benefits of data-driven results, and best practices for effective collaboration with CRO services.
The Importance of CRO Services in Medical Research
Contract Research Organizations (CROs) are pivotal in advancing clinical research, providing a suite of services that propel studies from conception to conclusion. They bring a wealth of expertise in navigating the intricate landscape of clinical trials, managing vast datasets, and upholding stringent regulatory standards.
Collaborating with CROss offers researchers access to specialized skills and state-of-the-art facilities, which can markedly boost the efficiency and success rate of clinical endeavors. Moreover, CROs are adept at addressing the complex ethical, legal, and social challenges that surface in the development of emerging technologies.
They help delineate clear, SMART objectives—specific, measurable, attainable, relevant, and time-bound—tailoring their services to meet the precise needs of the research while leveraging internal resources effectively. The 2023 Perceptions and Insights Study by CISCRP underscores the critical role of research professionals in patient engagement and the broader mission of enhancing patient outcomes. As the medical community continues to tackle diseases on a global scale, the strategic partnership with CROs becomes increasingly essential, ensuring that the social goals of research are met and that every study is a step toward improving the human condition.
Challenges Faced in Medical Research and the Role of CRO Services
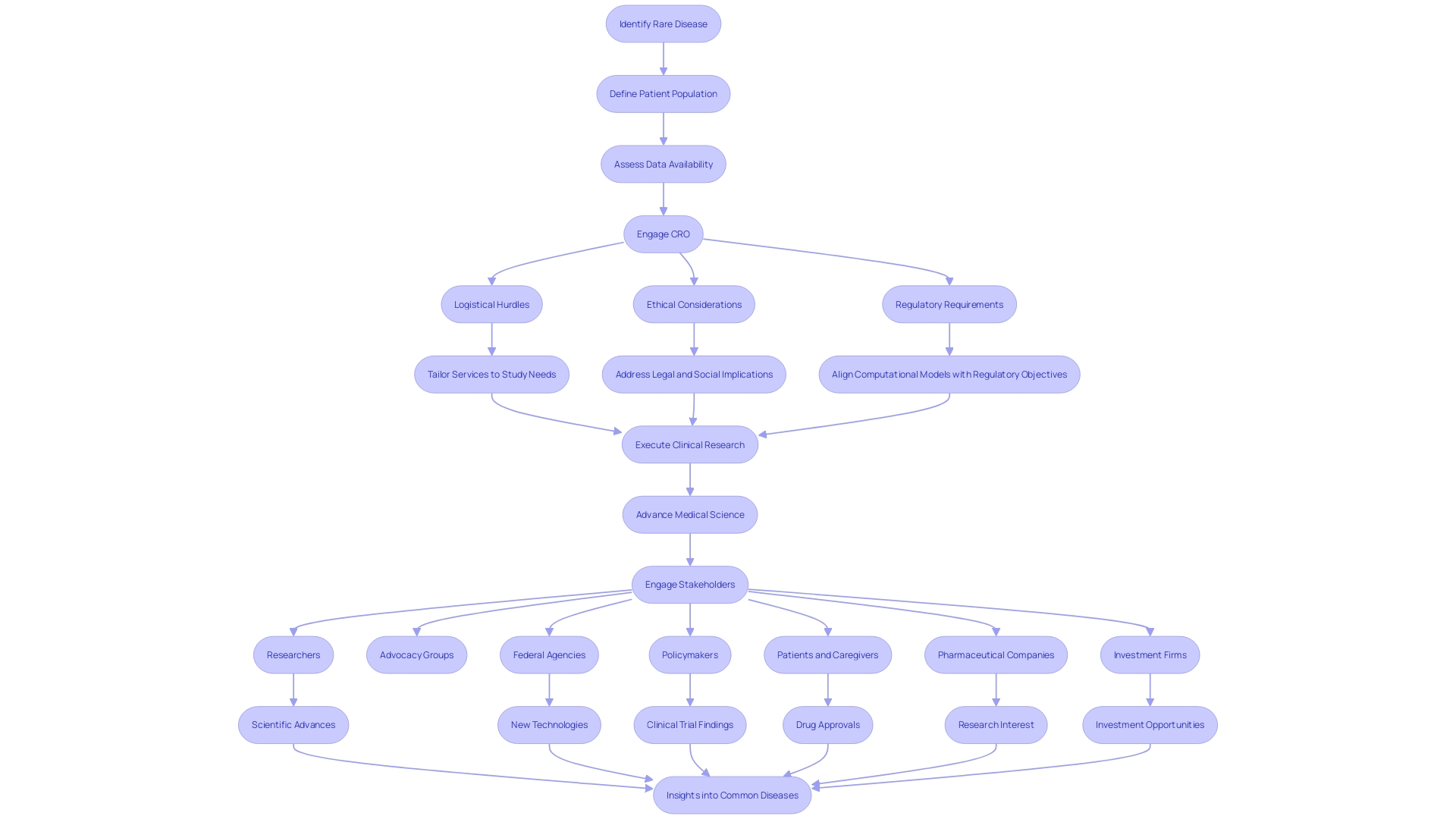
Navigating the complexities of clinical research, especially for rare diseases, requires meticulous planning and precise execution. The challenge is amplified when patient populations are scarce and dispersed, as illustrated by a patient in rural Pennsylvania with an ultra-rare condition contemplating participation in a clinical trial in Turkey. This scenario underscores not only the logistical hurdles of cross-border travel but also the intricate web of ethical, legal, and social issues that must be delicately balanced.
A Contract Research Organization (CRO) becomes instrumental in these instances, offering a network of investigators and research sites that can bridge the geographical and regulatory divides. By adopting a SMART approach to define specific, measurable, attainable, relevant, and time-bound requirements, CROss can tailor their services to the unique needs of a study, ensuring that resources are optimized and that any in-house capabilities are effectively leveraged. Moreover, CROss are positioned to address the Question(s) of Interest and Context of Use, ensuring that the computational models and other sources of information are aligned with the regulatory submission's objectives.
The value of a CRO in this setting is multi-faceted. They ensure adherence to the evolving international legal and regulatory landscape, address the social goals of the research aimed at improving the human condition, and consider the technology's ethical implications. This holistic approach not only enhances the reliability of research outcomes but also resonates with a broad spectrum of stakeholders, including researchers, advocacy groups, federal agencies, and investors, all of whom are invested in the advancement of medical science for both rare and common diseases.
Case Study: Successful Implementation of CRO Services in Medical Research
A medical research organization aiming to pioneer a first-in-human clinical trial for an innovative therapy encountered the intricate regulatory landscape of Latin America. With unique healthcare systems across its nations, where public, private, and social security services intertwine, successful navigation of this environment is critical.
To address this challenge, the organization collaborated with a Contract Research Organization (CRO) well-versed in the region's clinical trial protocols. This partnership was pivotal in steering through the regulatory complexities, as Latin America's healthcare systems, though offering basic cancer care, often present barriers to accessing high-cost drugs, and variability in service availability is common.
The CRO's expertise in primary research, evidence synthesis, and policy analysis, akin to the skills highlighted by Kati from Economist Impact, was instrumental in site selection and ensuring the trial adhered to regulatory and ethical standards. As a result, the trial was executed efficiently, meeting the required timelines and contributing to the collective advancement of medical science in the region. This case exemplifies the practical application of implementation science and the importance of leveraging local knowledge and scientific evidence to enhance the quality and safety of neonatal care, as noted in the study published in Enfermagem 2023.
Benefits of CRO Services: Data-Driven Results and Insights
Contract Research Organizations (CROs) are pivotal in advancing clinical research through their meticulous data management and statistical analysis capabilities. By employing these strengths, CROss not only ensure the accuracy and integrity of data collection but also enhance the analysis, driving insights that guide informed decision-making. The 2023 Perceptions and Insights Study by CISCRP highlights the significance of research professionals in patient engagement, recruitment, and retention, emphasizing the human element in clinical trials.
CROss contribute to this by providing access to extensive patient databases, which enhances the diversity of patient populations studied and strengthens the external validity of research outcomes. As research teams, often operating with limited internal resources, focus on defining their specific, measurable, attainable, relevant, and time-bound (SMART) requirements, CROss can offer tailored services that fill gaps and complement in-house capabilities. This symbiotic relationship between research professionals and CROss is essential in fostering the collective aim of improving patient lives on both individual and community levels.
Best Practices for Effective Collaboration with CRO Services
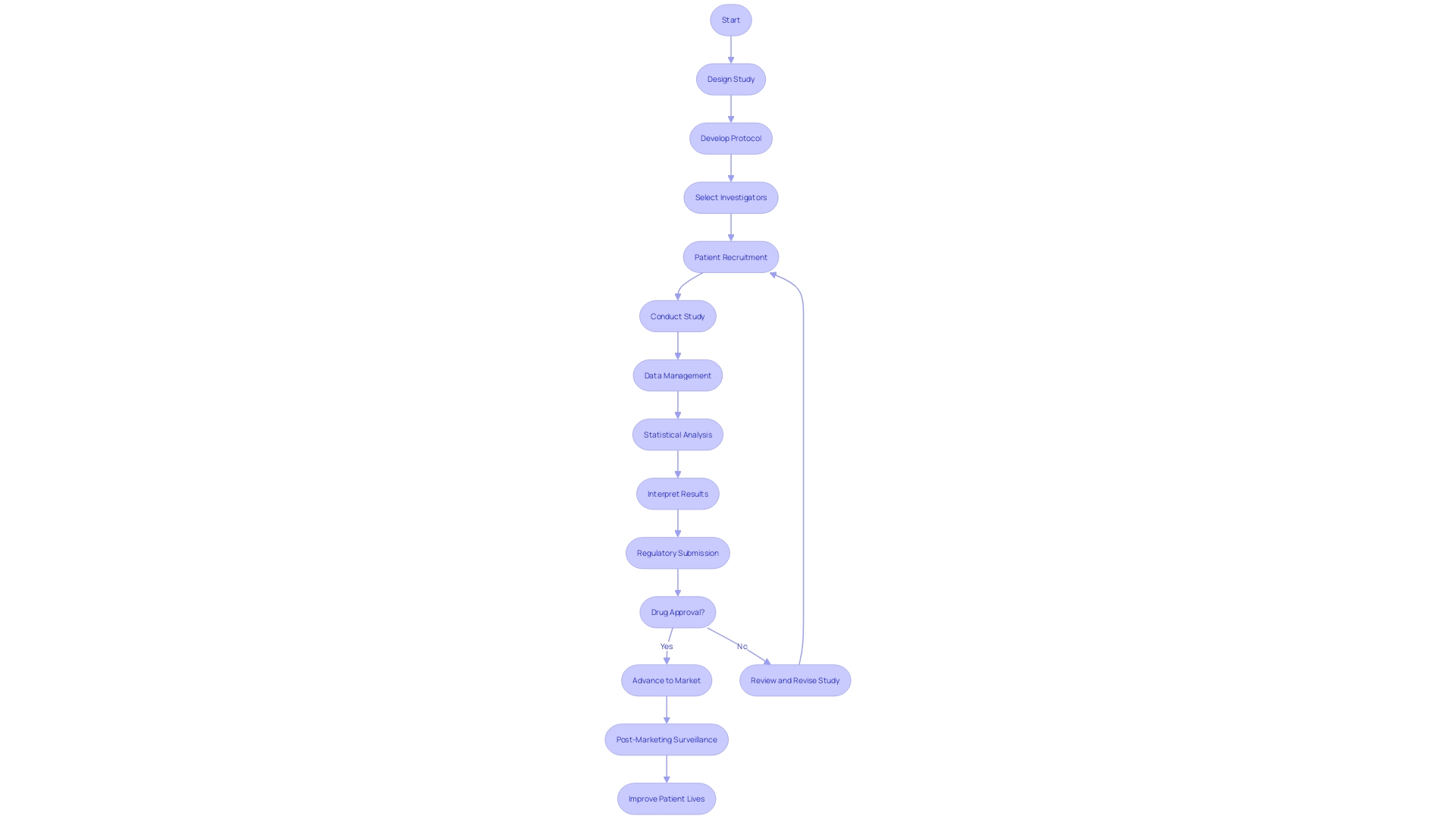
Enhancing research outcomes through collaboration with Contract Research Organizations (CROs) necessitates a strategic approach that is both systematic and adaptive. To achieve this, researchers must first delineate their requirements using the SMART framework—ensuring goals are Specific, Measurable, Attainable, Relevant, and Time-bound.
This precision in planning allows for a clear presentation of needs, leading to accurate proposals and efficient resource utilization. Furthermore, it is imperative to assess which aspects of the research can be managed internally, considering the lean nature of many clinical teams.
The collaboration should be characterized by open communication, fostering an environment where updates are exchanged regularly, and any arising challenges are promptly addressed. This is in line with the principles of interdisciplinary collaboration, which is known to yield more comprehensive solutions by integrating various areas of expertise, such as medicine, engineering, and social sciences.
An established project management plan, which includes detailed timelines, milestones, and deliverables, is essential to maintaining a streamlined research process and navigating the complex landscape of healthcare research and innovation. A successful partnership with a CRO also hinges on a transparent and cooperative relationship, underpinned by mutual trust and shared objectives. By leveraging the diverse perspectives and expertise of all parties involved, research can transcend traditional boundaries, leading to impactful healthcare innovations that consider medical efficacy, user experience, and psychological well-being. The importance of such collaboration is further emphasized by contributions to policy, influences on care practices, and the development of health services and products, as evidenced by collaborative research initiatives such as the ELSA study funded by Diabetes UK and the Juvenile Diabetes Research Foundation.
Conclusion
In conclusion, Contract Research Organizations (CROs) are vital in advancing medical research. They bring expertise in clinical trials, data management, and regulatory compliance, enhancing efficiency and success rates. Collaborating with CROs provides access to specialized skills and facilities, essential for tackling global diseases and improving patient outcomes.
CROs navigate the complexities of clinical research, especially for rare diseases and dispersed patient populations. Their SMART approach tailors services to study needs, optimizing resources and ensuring regulatory alignment. Successful case studies showcase CRO services overcoming regulatory complexities, accelerating trials, and contributing to medical science advancement.
Data-driven results and insights from meticulous data management enhance decision-making and strengthen research outcomes. Effective collaboration with CROs requires strategic planning, open communication, interdisciplinary collaboration, and a project management plan. Transparent relationships based on trust and shared objectives drive successful partnerships.
In summary, researchers partnering with CROs advance medical research by leveraging specialized skills and data-driven insights. This collaboration is crucial for addressing complex challenges while improving patient outcomes. By optimizing resources and navigating regulatory complexities, CROs play a pivotal role in advancing medical science on a global scale.




