Overview
The article emphasizes the critical importance of enhancing patient diversity in Argentine clinical trials, a necessity for improving the generalizability and effectiveness of medical research findings. Diverse participation is not merely beneficial; it is essential for identifying variations in drug metabolism across different demographic groups. The article addresses significant challenges, including historical distrust and logistical barriers that hinder inclusivity. To overcome these obstacles, it proposes strategic solutions such as:
- Community engagement
- Regulatory support
This collaboration is vital for advancing the quality and applicability of clinical research outcomes.
Introduction
In the realm of clinical trials, patient diversity emerges as a pivotal factor that shapes the efficacy and applicability of medical research. The evolving landscape of healthcare underscores the necessity of including participants from varied backgrounds—spanning different ethnicities, ages, and health statuses. This relevance is particularly pronounced in Argentina, where the rich tapestry of its population necessitates a more inclusive approach to clinical research. By embracing diversity, trials yield results that resonate with the broader community and pave the way for personalized medicine that addresses the unique needs of different demographic groups.
However, achieving this diversity presents challenges, from historical mistrust to socioeconomic barriers. This article delves into the significance of patient diversity in clinical trials, identifies the obstacles faced in Argentina, and outlines strategic approaches to enhance recruitment and foster community engagement, ultimately aiming to create a more equitable healthcare landscape.
Understand the Importance of Patient Diversity in Clinical Trials
Patient diversity in Argentine clinical trials is essential for several reasons. Firstly, it significantly enhances the generalizability of study results. By including participants from diverse backgrounds—spanning various ethnicities, ages, genders, and health statuses—the findings become more applicable to the broader population. This is particularly crucial in Argentina, where the ethnic and socioeconomic diversity of the population influences patient representation in clinical trials. Secondly, diverse participation aids in identifying potential differences in drug metabolism and efficacy across demographic groups, leading to more tailored and effective treatments. Research indicates that certain medications can exhibit varying effects based on genetic backgrounds, underscoring the necessity for inclusive research practices.
Moreover, patient diversity in Argentine clinical trials enriches the variety of research studies, promoting fairness in healthcare and ensuring that all populations benefit from advancements in medical science. For instance, the FDA has issued over 40 pre-notices of noncompliance to encourage voluntary adherence to reporting obligations, highlighting the regulatory focus on diversity in research studies. Additionally, the involvement of individuals over 65 in studies has increased from 10 percent in 2014 to 39.9 percent in 2020, suggesting a positive trend towards broader representation in research groups.
The challenges in presenting demographic information in research studies have revealed shortcomings that hinder the examination of inclusion trends, emphasizing the need for improved reporting methods. By acknowledging these factors, stakeholders can better appreciate the importance of fostering diversity in research, ultimately enhancing the quality and significance of outcomes. The key findings of the PLATINUM Diversity research, published in JAMA Cardiology, further illustrate the ongoing efforts to address inclusion in medical studies. Furthermore, media attention from Clinical Leader on research studies in Latin America, including Colombia, highlights the growing recognition of this issue. Bioaccess's comprehensive management services for studies, including feasibility assessments and site selection, play a vital role in promoting patient diversity in Argentine clinical trials, ensuring that studies are designed to be inclusive and representative of the populations they aim to serve.

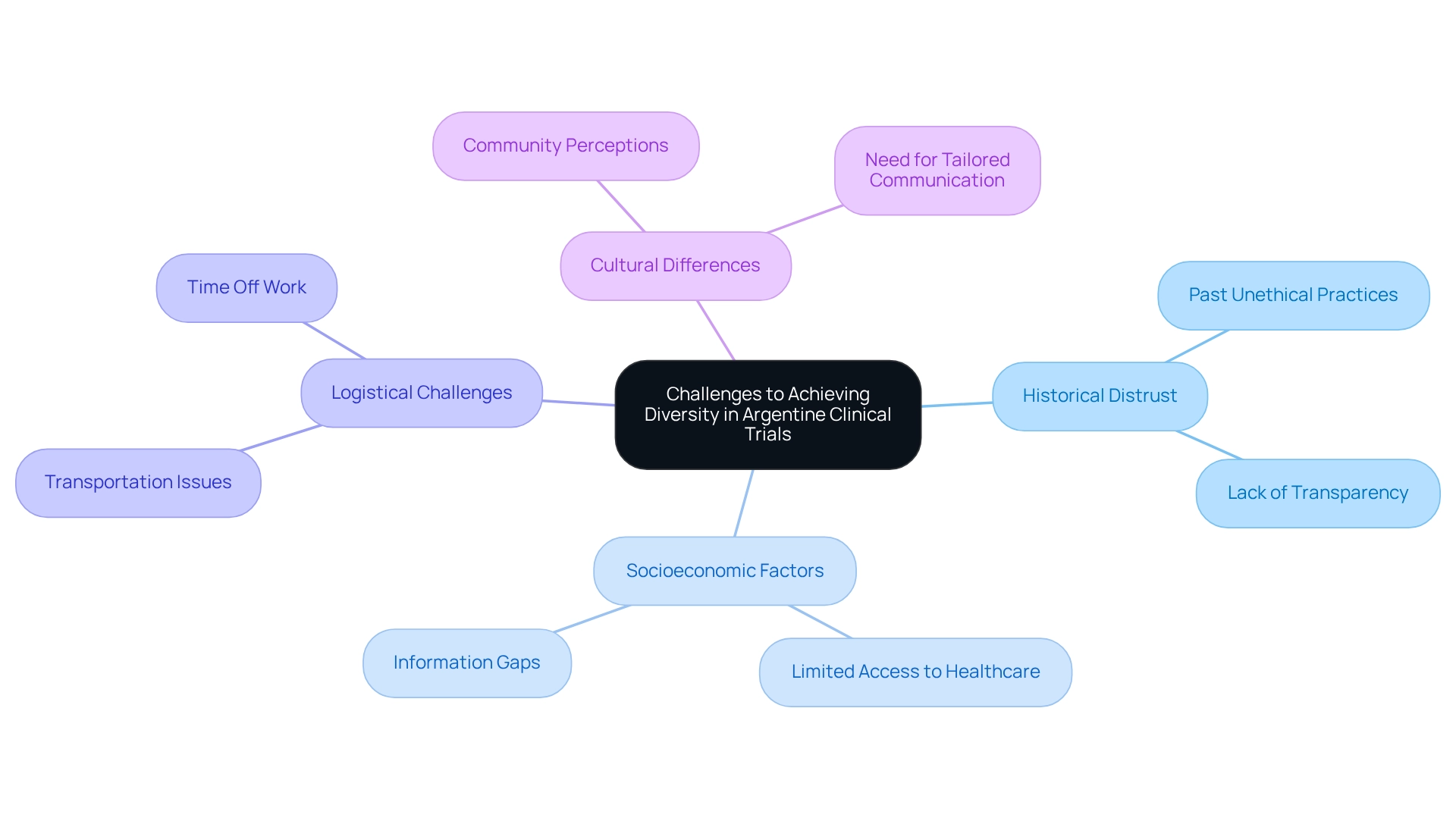
Identify Challenges to Achieving Diversity in Argentine Clinical Trials
The challenges of obtaining variety underscore the critical importance of patient diversity in Argentine clinical trials. A primary obstacle is the historical distrust of medical research within marginalized communities, rooted in past unethical practices and a pervasive lack of transparency in research procedures. Research indicates that Black Americans are frequently undertreated for pain compared to white patients, highlighting the broader issue of health inequalities that can affect participation in research studies. This distrust is exacerbated by socioeconomic factors; individuals from lower-income backgrounds often face limited access to healthcare and information regarding research studies, which diminishes their chances of involvement.
Logistical challenges, including transportation issues and the necessity of time off work, further complicate recruitment efforts. Additionally, cultural differences can influence community perceptions of research studies, emphasizing the need for tailored communication strategies that resonate with diverse groups. For example, Sanofi is forming a coalition of non-profits, activists, businesses, and governments to collaborate on research and disseminate best practices aimed at addressing health disparities.
Recognizing these multifaceted challenges empowers study sponsors to develop more inclusive recruitment strategies, ultimately fostering greater participation from underrepresented groups and enhancing patient diversity in Argentine clinical trials. Organizations like bioaccess® leverage their expertise in comprehensive research management services—including feasibility studies, site selection, compliance reviews, setup, and project management—to effectively address these challenges. By doing so, they not only expedite the development of medical devices but also contribute to improving inclusion in research studies, which is essential for advancing global health through international cooperation and innovation in Medtech.
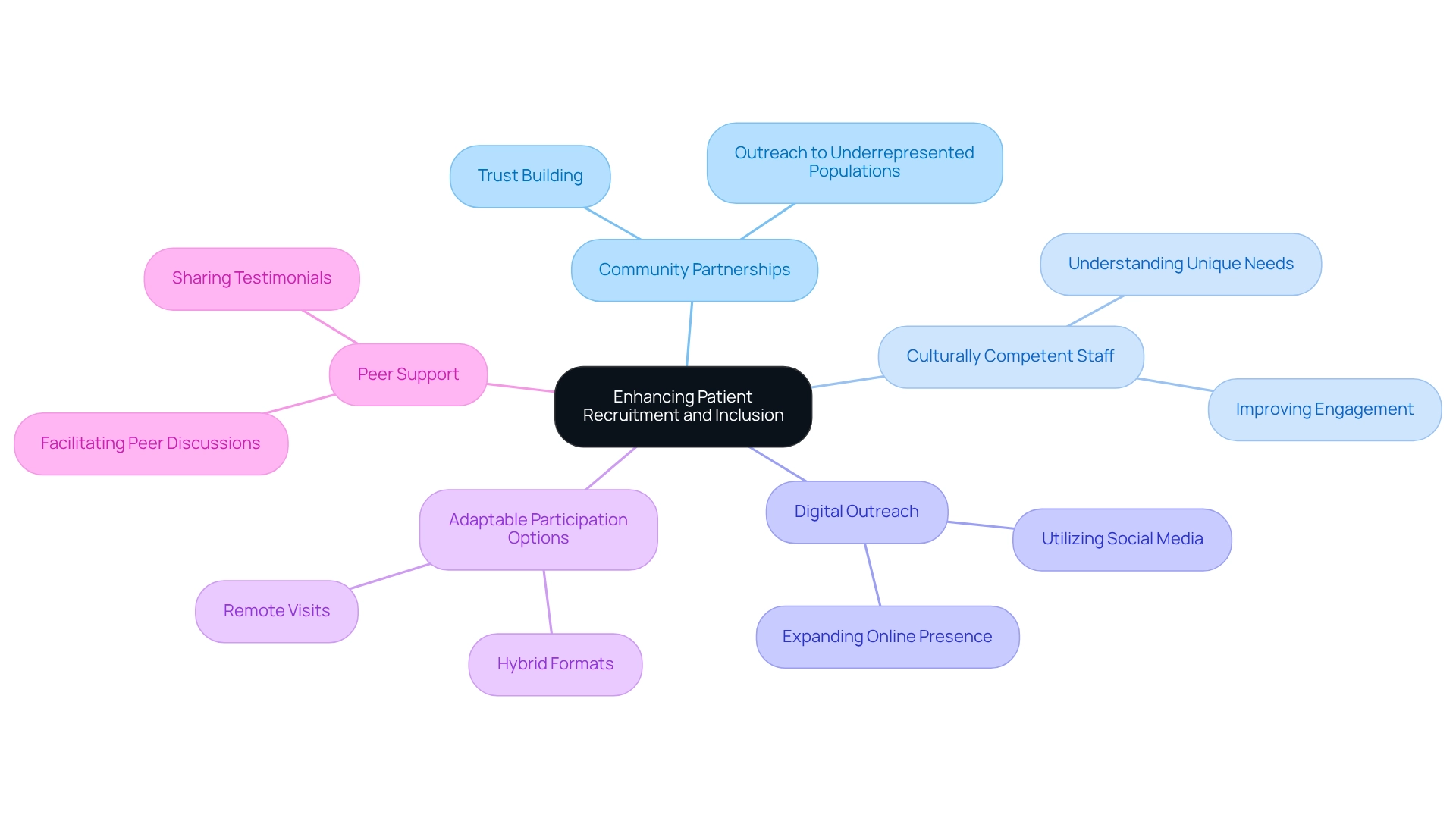
Implement Strategies to Enhance Patient Recruitment and Inclusion
Improving patient recruitment and involvement in research studies necessitates a multifaceted strategy. Establishing partnerships with community organizations is crucial; these entities foster trust and facilitate outreach to underrepresented populations. Their established relationships within the community provide insights into effective communication strategies tailored to specific demographics.
Statistics indicate a notable absence of variation in medical studies. For example, in cancer treatment research conducted between 2001 and 2010, 82.9% of participants were white, while only 6.2% were African American, 3.3% were Asian, 2.2% were Hispanic, and 0.1% were Native American. This highlights the significance of applying strategies that improve patient diversity in Argentine clinical trials.
Employing culturally competent staff is another vital strategy. These professionals understand the unique needs and concerns of diverse populations, significantly improving participant engagement. Additionally, leveraging digital platforms and social media expands outreach efforts, particularly among younger demographics who are more active online. As noted by Antidote, 'Employing tools and technology that decrease the number of site visits — like electronic patient-reported outcomes (ePRO) and home visits — may enhance enrollment for the patients that study teams find most challenging to reach.'
Providing adaptable involvement choices, such as remote visits or hybrid formats, can further lower obstacles for potential participants, simplifying their engagement with research studies. Clear communication about the benefits and risks of participation, along with compensation for time and travel, serves as an incentive for involvement.
Moreover, the case study titled 'The Importance of Peer Support in Research Studies' emphasizes that patients, particularly those with cancer, appreciate the chance to engage with peers who have participated in research activities. Facilitating peer discussions and testimonials can boost trust and motivate more patients to consider joining research studies.
By applying these approaches, health studies can enhance patient diversity in Argentine clinical trials, leading to increased variety and inclusiveness, ultimately resulting in more representative and effective findings.
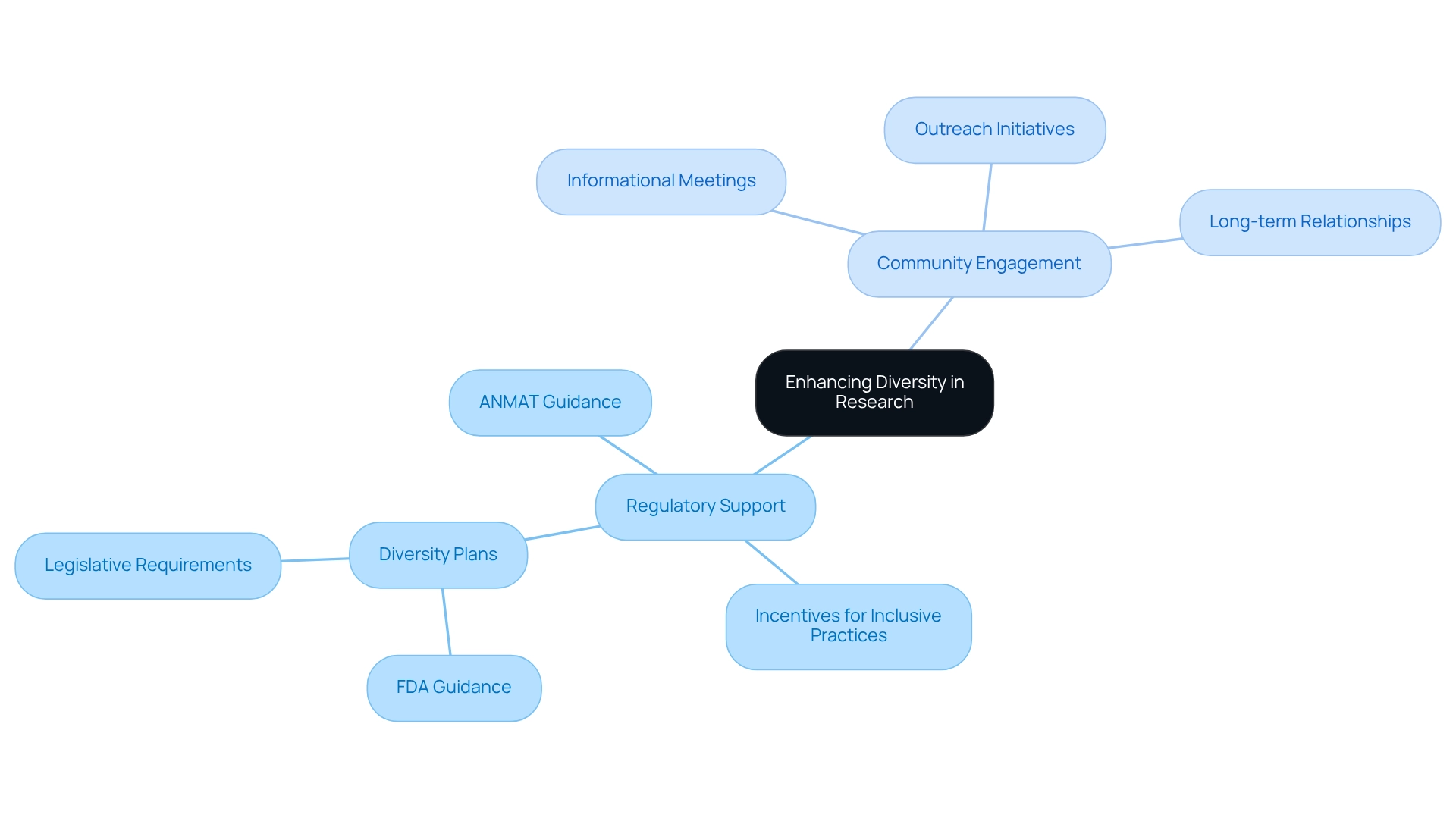
Leverage Regulatory Support and Community Engagement for Diversity
Utilizing regulatory assistance and community involvement is crucial for enhancing diversity in research studies. Regulatory entities, such as ANMAT in Argentina, are progressively recognizing the significance of patient diversity in Argentine clinical trials. They provide guidance and potential incentives for initiatives that emphasize inclusive practices. For instance, authorizations for facilities conducting Phase I studies will be valid for five years, underscoring the importance of timely and comprehensive study designs that align with national health priorities. By aligning study objectives with these priorities, sponsors can enhance the relevance and impact of their research.
At bioaccess®, we bring over 20 years of experience in Medtech, offering comprehensive clinical trial management services that include:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Reporting
Our expertise in managing Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF) ensures that diversity is a fundamental aspect of our clinical research practices.
Community involvement efforts, such as informational meetings and outreach initiatives, play a vital role in educating potential participants about the significance of research studies and their contributions to medical progress. Establishing long-term relationships with community leaders and stakeholders fosters ongoing dialogue and trust, which are essential for effective recruitment efforts. Effective community engagement approaches demonstrate that when local populations are informed and involved, participation rates in clinical studies significantly rise. For example, initiatives that actively involve community members in the research process have been shown to improve recruitment and retention rates.
Furthermore, the evaluation of the FDA's diversity plan guidance emphasizes the necessity for regulatory organizations to strengthen the importance of diversity plans, particularly in light of new legislative mandates that may render these plans compulsory for phase 3 studies. As FDA Commissioner Robert Califf stated, "The agency’s draft guidance is a significant move—and one of many continuous initiatives—to tackle the involvement of underrepresented groups in research studies to enhance the information we possess regarding patients who will utilize the medical products if authorized."
By merging regulatory assistance with robust community involvement strategies, trials can foster patient diversity in Argentine clinical trials, benefiting all stakeholders and ultimately resulting in more representative data and improved health outcomes. Our customized approach at bioaccess® aims to advance medical devices sooner while ensuring that diversity remains a core principle in our clinical research practices.
Conclusion
The significance of patient diversity in clinical trials is paramount, particularly in a diverse country like Argentina. By incorporating participants from various backgrounds, clinical trials yield results that are more applicable to the general population, ultimately paving the way for more effective and personalized medical treatments. Addressing the challenges that hinder diversity—such as historical mistrust, socioeconomic barriers, and cultural differences—requires tailored strategies and robust community engagement.
Efforts to enhance recruitment and inclusion are essential. Establishing partnerships with local organizations, employing culturally competent staff, and leveraging digital platforms can forge pathways for improved engagement with underrepresented populations. Furthermore, offering flexible participation options and communicating the benefits of involvement clearly can further incentivize participation in clinical trials.
Regulatory support and community engagement are vital components in cultivating a more inclusive clinical research landscape. By aligning trial objectives with national health priorities and fostering trust within communities, clinical trials can achieve greater diversity, leading to more representative and impactful research outcomes. Ultimately, prioritizing diversity in clinical trials not only elevates the quality of medical research but also contributes to a more equitable healthcare system that benefits all demographic groups.
Frequently Asked Questions
Why is patient diversity important in Argentine clinical trials?
Patient diversity is essential in Argentine clinical trials because it enhances the generalizability of study results, making findings more applicable to the broader population. It also helps identify differences in drug metabolism and efficacy across demographic groups, leading to more tailored and effective treatments.
How does Argentina's population diversity influence clinical trials?
Argentina's ethnic and socioeconomic diversity influences patient representation in clinical trials, making it crucial to include participants from various backgrounds to ensure the results reflect the entire population.
What are the benefits of including diverse participants in clinical research?
Including diverse participants in clinical research promotes fairness in healthcare, ensures that advancements in medical science benefit all populations, and enriches the variety of research studies.
What trends have been observed regarding the inclusion of older individuals in clinical studies?
The involvement of individuals over 65 in clinical studies has significantly increased from 10 percent in 2014 to 39.9 percent in 2020, indicating a positive trend towards broader representation in research groups.
What challenges exist in presenting demographic information in research studies?
There are challenges in presenting demographic information that hinder the examination of inclusion trends, highlighting the need for improved reporting methods to better understand and foster diversity in research.
What role do organizations like Bioaccess play in promoting patient diversity?
Bioaccess provides comprehensive management services for studies, including feasibility assessments and site selection, which are vital in promoting patient diversity in Argentine clinical trials and ensuring studies are inclusive and representative of the populations they aim to serve.




