Introduction
In the intricate landscape of medical device regulation, post-market clinical follow-up (PMCF) studies serve as a pivotal mechanism to monitor ongoing safety and efficacy. These studies are indispensable, particularly after a device has navigated the complex regulatory pathways to market entry.
For instance, in the United States, devices are categorized into three classes by the FDA, each reflecting a different level of patient risk. This classification determines the subsequent registration process, whether it be a 510(k) premarket notification, a more stringent premarket approval (PMA), or the De Novo process for novel devices without existing predicates.
Once a medical device is FDA cleared, approved, or granted under the De Novo process, PMCF studies become essential in capturing real-world data. This continuous evaluation encompasses the identification of any device-related adverse events, use errors, or product problems, which are critical for informing both healthcare professionals and regulatory bodies. The data collected, including device type, manufacturer details, and any incidents of malfunction or defects, provide insights that are vital for safeguarding patient health and guiding future regulatory actions. As the FDA oversees a broad spectrum of public health responsibilities, the role of PMCF studies in ensuring the integrity of medical devices cannot be overstated, offering a lens into the device's performance beyond the controlled environment of pre-market trials.
Understanding the Importance of Safety and Efficacy
In the intricate landscape of medical device regulation, post-market clinical follow-up (PMCF) studies serve as a pivotal mechanism to monitor ongoing safety and efficacy. These studies are indispensable, particularly after a device has navigated the complex regulatory pathways to market entry.
For instance, in the United States, devices are categorized into three classes by the FDA, each reflecting a different level of patient risk. This classification determines the subsequent registration process, whether it be a 510(k) premarket notification, a more stringent premarket approval (PMA), or the De Novo process for novel devices without existing predicates.
Once a medical device is FDA cleared, approved, or granted under the De Novo process, PMCF studies become essential in capturing real-world data. This continuous evaluation encompasses the identification of any device-related adverse events, use errors, or product problems, which are critical for informing both healthcare professionals and regulatory bodies. The data collected, including device type, manufacturer details, and any incidents of malfunction or defects, provide insights that are vital for safeguarding patient health and guiding future regulatory actions. As the FDA oversees a broad spectrum of public health responsibilities, the role of PMCF studies in ensuring the integrity of medical devices cannot be overstated, offering a lens into the device's performance beyond the controlled environment of pre-market trials.
Key Documents for Clinical Evaluation: MDR and MDCG Guidelines
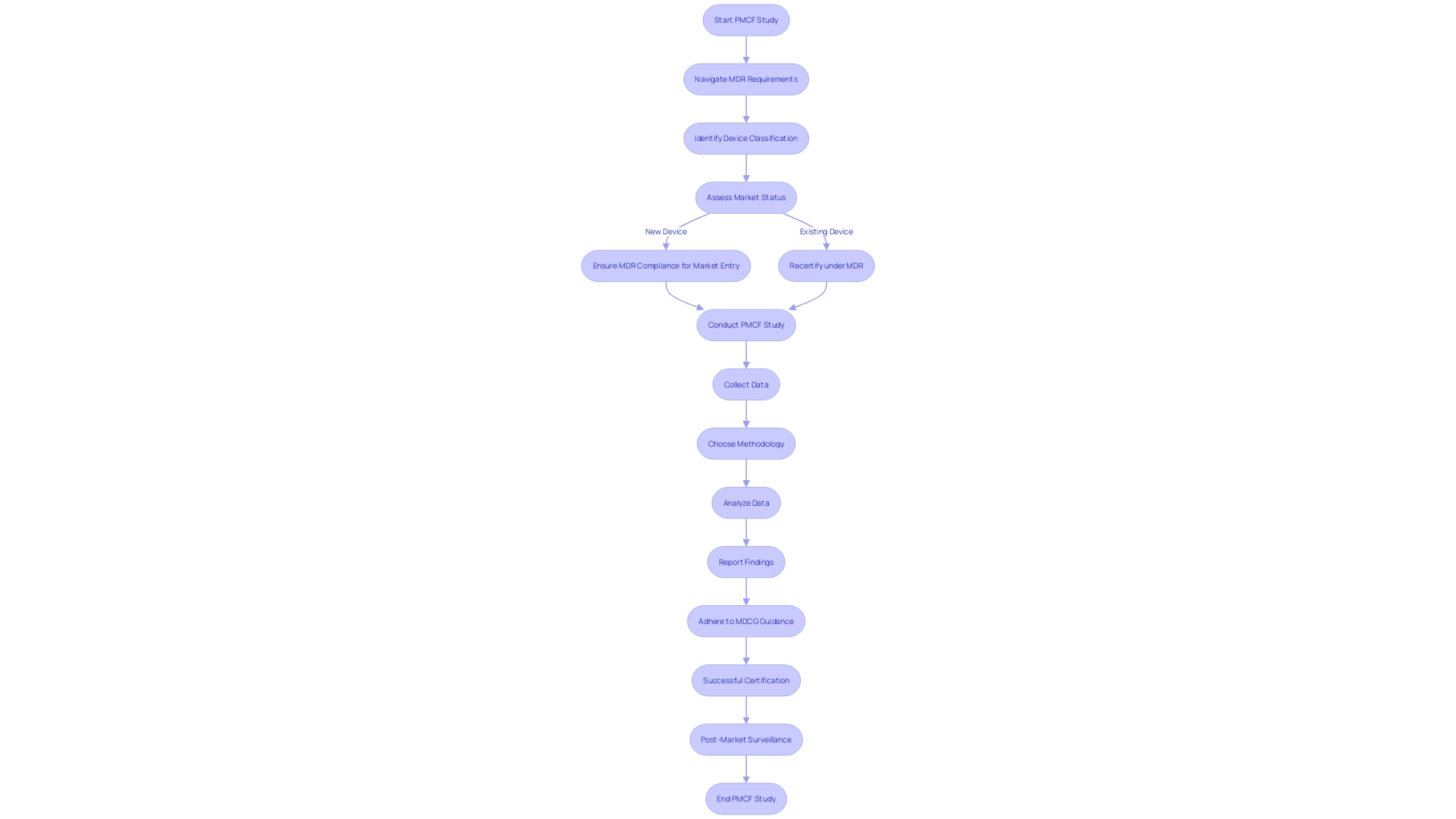
Post-market clinical follow-up (PMCF) studies are a critical component of medical device surveillance in Europe. Adhering to the Medical Device Regulation (MDR) and guidance by the Medical Device Coordination Group (MDCG) is not only a regulatory requirement but a commitment to patient safety. These guidelines articulate the necessary methodologies and data requirements for PMCF studies.
Familiarity with these regulations is crucial, as they have been significantly revised to encompass a broader range of products, including drug-device combinations, and devices with ancillary medicinal substances. This detailed guidance supports manufacturers in understanding their responsibilities for the lifecycle management of these complex products. Moreover, with the MDR introducing new requirements, it is imperative for manufacturers to ensure their devices comply with the updated legal framework, particularly for products already in the market during the transition period.
As regulatory partners, we assist in navigating these complex environments to achieve successful certification. With the EMA and national competent authorities taking on new roles in the assessment of certain medical device categories, the importance of stringent PMCF studies is underscored. These studies not only confirm the continued safety and efficacy of medical devices post-market but also play a pivotal role in the comprehensive regulatory scrutiny of medical devices in Europe.
The Role of Clinical Investigation Reports and Post-Marketing Surveillance
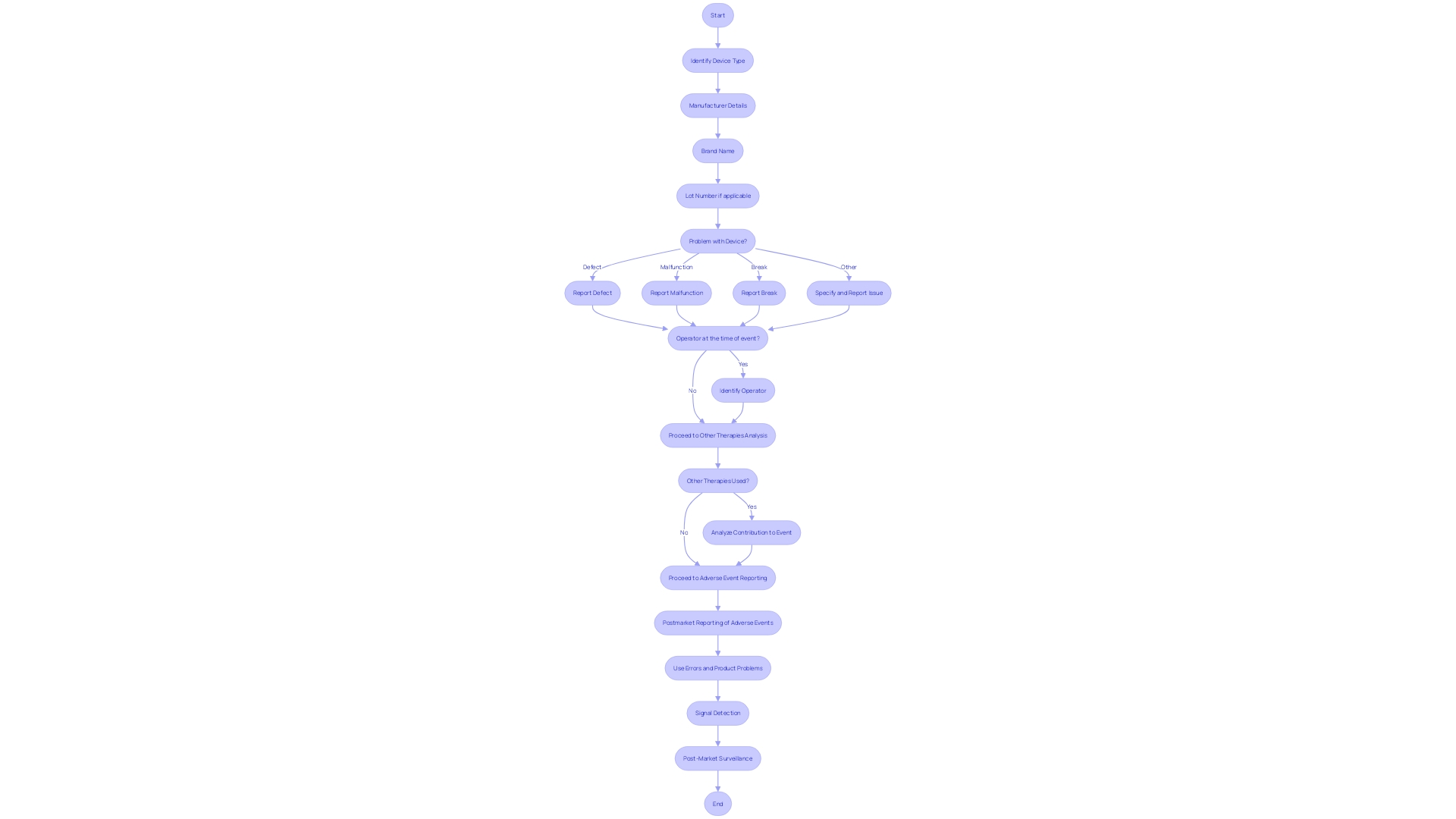
In the realm of medical devices, the safety and efficacy of products are paramount. Clinical Investigation Reports (CIRs) provide an essential analysis of post-market data, offering a detailed look into device performance through the lens of real-world application.
These reports meticulously detail the device type, manufacturer, brand name, and lot number, alongside the nature of any issues, such as defects or malfunctions. They also consider whether the device was in use during the event and if concurrent therapies might have influenced the outcome.
The importance of such data is underscored by the FDA's rigorous classification system, which assigns risk values to medical devices and dictates the pathway to market authorization—be it 510(k) Premarket Notification, Pre-Market Approval (PMA), or the De Novo process. Adverse event reporting and signal detection are integral components of post-market surveillance, feeding into a continuous cycle of safety monitoring. This proactive approach to capturing and analyzing post-market events ensures that any emerging risks are swiftly identified and addressed, thereby safeguarding patient well-being and upholding the device's integrity in the marketplace.
Ensuring Compliance with Regulatory Requirements
The intricate landscape of post-market clinical follow-up (PMCF) studies requires a multi-faceted approach to compliance, particularly in Latin America where the medical device market is rapidly evolving. Researchers and manufacturers are tasked with navigating a complex array of ethical, legal, and social considerations, as highlighted by case studies which delve into market incentives, intellectual property rights, and the broader impact of emerging technologies. These case studies, structured around pivotal questions, illuminate the pressing ethical dilemmas and regulatory gaps that can arise during the lifecycle of medical technologies.
To ensure that the safety and efficacy of medical devices are continually monitored, PMCF studies must align with international standards, respecting patient privacy, data protection, and informed consent protocols. Moreover, these studies must satisfy local regulations, which can be as diverse as the regions themselves. The FDA's role in assuring the public health by overseeing drugs, medical devices, and other health-related products serves as a benchmark for regulatory compliance, emphasizing the importance of safeguarding patient welfare and generating robust clinical evidence.
The development and qualification of Digital Health Technologies (DHTs) within clinical trials, as discussed in FDA public meetings, underscore the necessity for meticulous verification and validation processes. Just as the FDA has set forth guidance on DHTs for remote data acquisition in clinical investigations, Latin American markets must also strive for high standards in the development and utilization of such technologies to ensure data integrity and reliability. This is essential not only for regulatory compliance but also for the achievement of social goals aimed at enhancing the human condition through medical research.
Best Practices for Clinical Evaluation Reports (CERs)
In the realm of medical device safety and efficacy, Clinical Evaluation Reports (CERs) stand as a cornerstone, synthesizing post-market clinical data to affirm the ongoing reliability of these healthcare tools. The integrity of Cars hinges on meticulous adherence to a spectrum of best practices.
These include designing robust studies that align with the device's FDA classification—be it 510(k), PMA, or De Novo—and carefully determining sample sizes that reflect the device’s risk profile. Standardization is key in both data collection and analysis, ensuring that the outcomes can be universally interpreted and applied.
Moreover, transparent reporting is non-negotiable; this allows for the scrutiny of adverse events, use errors, and product problems, which must be meticulously documented including the device type, manufacturer, and specifics of the event. Recent regulatory updates by entities such as the FDA and EMA underscore the vital nature of these practices, as they navigate new territories like integral drug-device combinations and companion diagnostics. Ultimately, the generation of reliable clinical evidence through CERs is a critical step in safeguarding patient health and supporting the successful lifecycle of medical devices, from initial market entry to potential strategic alliances or acquisitions.
The Impact of Artificial Intelligence and Machine Learning on Medical Devices
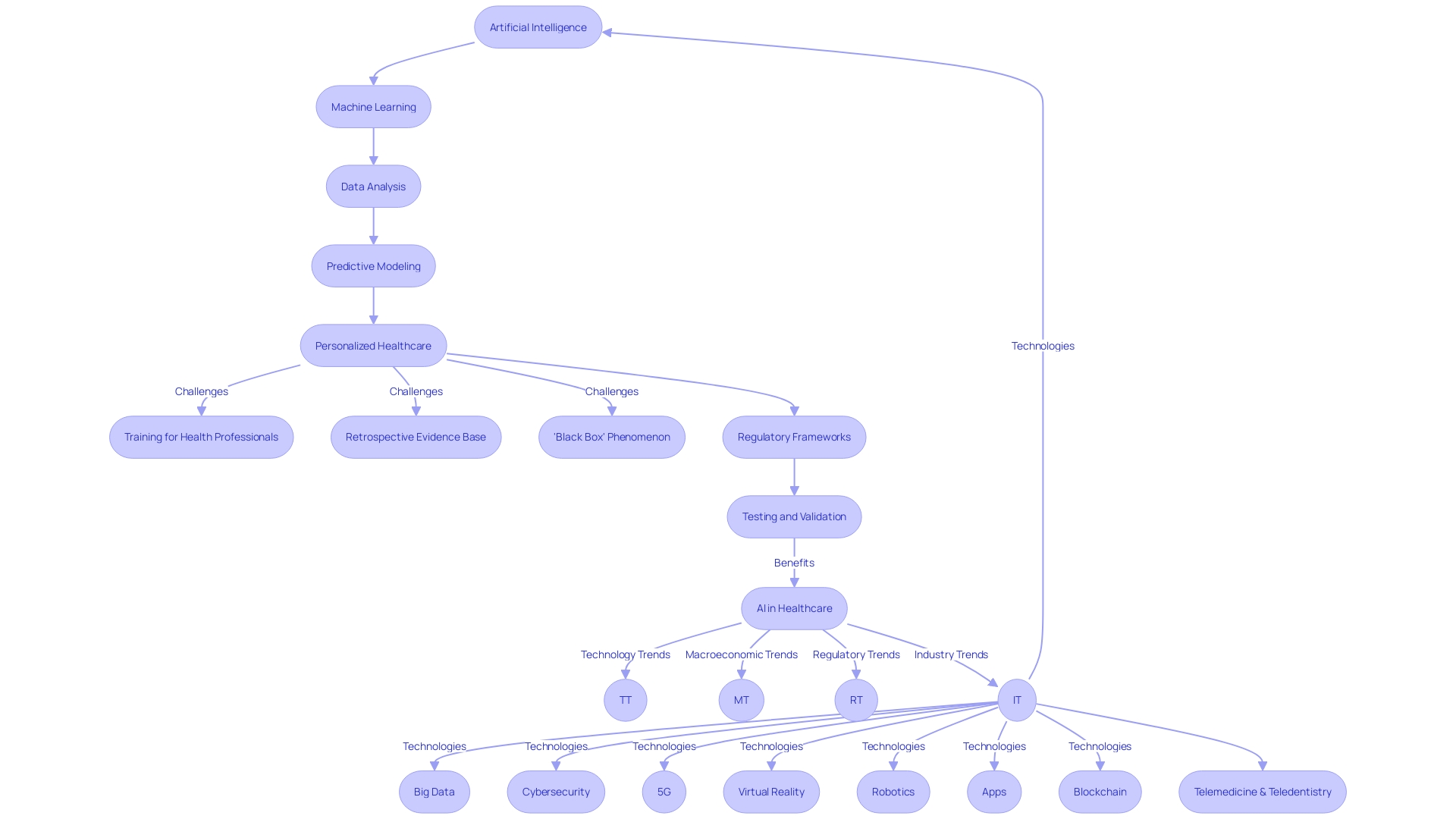
The integration of artificial intelligence (AI) and machine learning (ML) into the realm of medical devices is poised to significantly enhance patient care. These technologies bring the power of advanced data analysis and predictive modeling to the forefront, enabling a more personalized approach to healthcare.
With the capacity to sift through complex datasets from post-market clinical follow-up studies, AI and ML algorithms are instrumental in detecting trends and extracting insights regarding the performance of medical devices, patient outcomes, and possible risks associated with their use. However, the deployment of AI and ML in healthcare is not without its challenges.
Health professionals often lack the training required to effectively evaluate AI software, and the evidence base for AI applications in healthcare is largely retrospective, lacking in randomized controlled trials that clinicians can rely on for appraisal. The absence of rigorous pre-market evaluation can result in significant patient harm, a loss of confidence in the medical profession, and potentially misleading population-level conclusions.
Moreover, the 'black box' phenomenon, where the decision-making process of algorithms remains opaque, poses an additional hurdle to the acceptance and accountability of these technologies. The development of machine learning/artificial intelligence medical devices (MAMDs) parallels the drug development process, with a rigorous focus on efficacy and safety.
Yet, the success of these devices hinges not just on their technological sophistication, but also on their proven ability to improve patient outcomes. As such, there is a pressing need for regulatory frameworks that prioritize patient outcomes and mandate compliance with procedures that enhance healthcare quality. This is underscored by a recent White House executive order, which mandates the creation of AI-specific regulatory strategies by April 27, 2024. In light of the aging global population and the increasing burden on healthcare systems, AI and ML technologies offer a promising avenue to augment healthcare services. Studies have already demonstrated the efficacy of AI in improving case detection in breast cancer screening. As we move towards an 'AI-powered' healthcare future, it is paramount that AI and digital technologies undergo careful testing and real-world validation to ensure sustainable health systems that do not compromise the standards of care.
Conclusion
In conclusion, post-market clinical follow-up (PMCF) studies are crucial for monitoring the safety and efficacy of medical devices. These studies provide real-world data on device performance, identifying adverse events and product problems.
Compliance with regulatory requirements, such as the FDA classification system and the Medical Device Regulation (MDR) in Europe, is essential for ensuring patient safety. Clinical investigation reports (CIRs) analyze post-market data, offering valuable insights into device performance beyond pre-market trials.
Adverse event reporting and signal detection are integral components of post-market surveillance, enabling the swift identification of emerging risks. Best practices for Clinical Evaluation Reports (CERs) include designing robust studies aligned with device classification, standardizing data collection and analysis, and transparently reporting adverse events.
Reliable clinical evidence through CERs is critical for safeguarding patient health throughout the lifecycle of medical devices. The integration of artificial intelligence (AI) and machine learning (ML) in medical devices has the potential to enhance patient care through advanced data analysis.
However, challenges exist in evaluating AI software effectively and ensuring rigorous pre-market evaluation. Regulatory frameworks prioritizing patient outcomes and compliance procedures are necessary for an AI-powered healthcare future. Careful testing and validation are vital to ensure sustainable health systems without compromising standards of care. In summary, PMCF studies play a pivotal role in monitoring device safety and efficacy. Compliance with regulations, standardized reporting practices, and responsible incorporation of AI/ML technologies are essential for ensuring patient welfare and advancing healthcare innovation.




