Introduction
Medical device validation is a critical process that ensures the safety, efficiency, and reliability of medical devices. It involves rigorous testing, evaluation, and detailed documentation. From simple tools like bandages to advanced machines such as MRI scanners, each device presents unique challenges related to human and technological diversity.
The assessment of unobservable aspects, like pain and life quality, through Clinical Outcome Assessments (COAs), is crucial in determining a device's impact. Postmarket reporting plays a vital role in identifying defects or malfunctions. With the rapid innovation in the industry, validation processes and compliance programs must adapt to maintain stringent safety and quality standards.
Michigan's UL Solutions laboratory exemplifies the industry's focus on safety, security, usability, and interoperability. Thorough medical device validation is essential in safeguarding patient health and safety, mitigating risks, and ensuring excellence in product launches. It is a complex and multidimensional process that requires careful adherence to regulatory standards and a robust design control process.
By gathering clinical data and adhering to regulatory requirements, medical device manufacturers can gain market authorization and build a strong reputation in the competitive healthcare market. However, there are challenges to navigate, such as the diverse nature of medical devices and the need for extensive testing and evaluation. Overall, medical device validation plays a crucial role in ensuring the effectiveness and reliability of healthcare technology.
What is Medical Device Validation?
Validating medical devices is a multidimensional process that secures their safety, efficiency, and reliability. This vital process not only requires rigorous testing and evaluation but also detailed documentation. It is a complex field that embraces a vast array of devices, from simple tools like bandages to advanced machines such as MRI scanners, each with unique challenges related to human and technological diversity.
Clinical Outcome Assessments (Coas) play a significant role, capturing reports from direct sources like patients, which are paramount in assessing the unobservable aspects such as pain and life quality. Meanwhile, constant vigilance in postmarket reporting helps identify any device defects or malfunctions, ensuring ongoing safety measures. Medical device testing facilities, such as the new UL Solutions laboratory in Michigan, epitomize the industry's dynamism, focusing on safety, security, usability, and interoperability, which are essential aspects in a landscape where rapid innovation and risk management must coexist harmoniously.
Importance of Medical Device Validation
Medical device validation is crucial in safeguarding patient health and safety. The process diligently assesses devices to confirm their effectiveness and reliability, thus mitigating potential risks. Regulatory oversight, primarily by the FDA in the U.S. and by national authorities alongside EMA in Europe, classifies devices based on risk and dictates the level of scrutiny.
Notably, class three devices, which support or sustain life, go through more stringent reviews owing to their elevated risk profiles.
The pressing need for thorough device validation is underscored by historical oversight failures. As highlighted by the Philips Respironics case, where a sleep therapy device resulted in numerous injuries and fatalities, robust testing and transparent reporting are vital. Early, such devices could reach the market with minimal human testing, revealing lacunae in premarket evaluations and post-market surveillance.
Moreover, companies excelling in product launches, integrating software and hardware, demonstrate their ability to navigate this intricate space, garnering a competitive edge. As medical device technology evolves with breakthroughs like AI and user-friendly interfaces, adapting compliance programs and validation processes becomes imperative. Efficient, modern methods not only catalyze innovation but also help maintain stringent safety and quality standards.
Leaders in industry, like UL Solutions, recognize the escalating demand for advanced testing services. Michigan is emerging as a hub for these technological leaps, reflecting the state's significant expertise and manufacturing capabilities. By converging at forums like the Medical Device Manufacturing & R&D Summit, professionals continue to push the frontiers, exchanging knowledge and best practices that shape the future of patient care.
Steps in Medical Device Validation
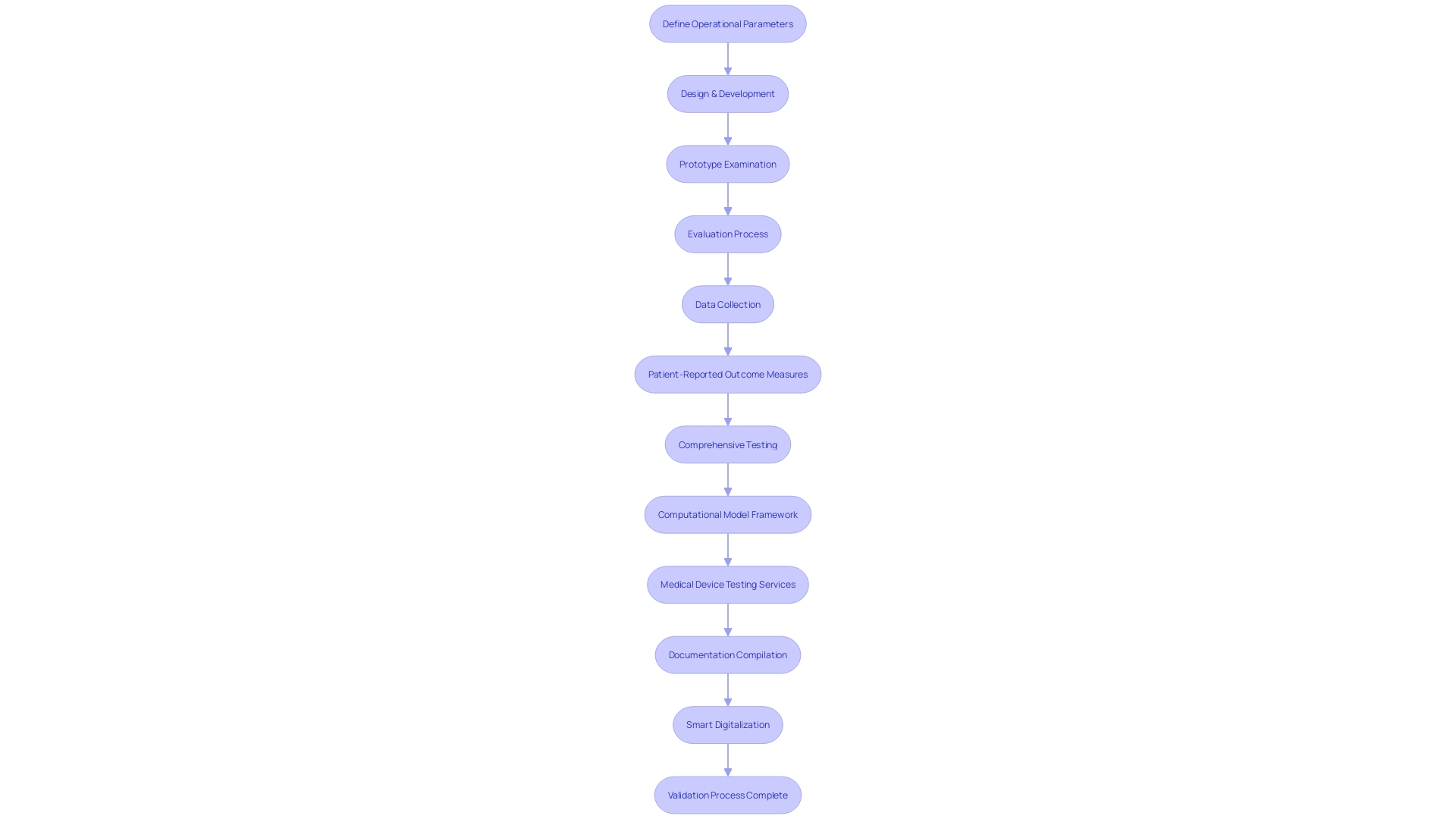
The pathway to validating medical device technology intertwines a sequence of rigorous steps aimed at verifying its safety, performance, and compliance with stringent regulatory standards. At the onset, a meticulous definition of the device's operational parameters and requirements is crafted, encompassing its designated purpose, performance criteria, and obligatory regulatory compliance elements.
Following the establishment of precise specifications, the design and development process culminates with the creation of a prototype, which then endures a thorough examination. This examination scrutinizes a gamut of attributes such as functionality, reliability, and overall performance. The meticulous evaluation process includes outcomes reported by a range of stakeholders, including clinicians, patients, non-clinical observers, or performance-based assessments, all converging to assess how the device influences patient health, function, or survival.
Data emanating from these evaluations must not only quantify scores or outcomes but also embody a transparent explanation of the methods, administration instructions, data collection standardization, scoring tactics, and interpretation of results. Moreover, a report from the patient, known as a Patient-reported outcome (PRO) measure, is notably significant for capturing the nuances of patients' health conditions directly, devoid of third-party interpretation. By focusing on symptoms and other intangible concepts such as pain severity and quality of life, PRO measures offer a unique and invaluable perspective on the device's impact.
In parallel, the documentary 'The Bleeding Edge' unveiled the discrepancies within the FDA's 510(k) clearance process, spotlighting instances where rigorous clinical trials were circumvented, occasionally leading to adverse patient outcomes. This revelation underscores the importance of comprehensive testing and validation for medical devices.
As the preparatory stages progress, a Computational Model Framework may be instituted, defining how the model will be utilized and the associated risks. This framework guides the risk assessment and defines the Context of Use (COU) – outlining the model's role in addressing the regulatory questions at hand. It arms the regulatory submission with a structured, question-focused approach grounded in the model's application.
To strengthen the validation process further, UL Solutions launched medical device testing services at their Rochester Hills, Michigan laboratory. This initiative caters to escalating demand, empowering manufacturers to elevate the safety, security, and interoperability of medical devices. Mary Joyce, vice-president and general manager of UL Solutions' mobility and critical systems group, praised Michigan's burgeoning medical device sector, hailing it as a national leader in talent, workforce, and manufacturing within the industry.
Building on this infrastructure, thorough documentation bridging validation protocols, comprehensive reports, and any requisite regulatory submissions is meticulously compiled. This documentation forms the crux of the validation process, serving as a testament to meeting predefined acceptance criteria and ultimately facilitating the medical device's pathway to market.
Understanding the nuances between the phrases 'Registered, Cleared, Approved, and Granted' is pivotal within the medical device industry. With devices classified into three tiers predicated on patient risk, and corresponding regulatory pathways of 510(k), PMA, or De Novo, it is essential to navigate these terms deftly to achieve FDA Clearance, Approval, or Grant for the commercialization of medical devices in the United States.
The significance of smart digitalization within medical device validation cannot be understated. It not only entails the transition of documentation to a digital format but also the discernment of critical data that informs the broader context of the device's lifecycle management. As the industry continues to evolve, initiatives like UL Solutions' testing services and advances in smart digitalization further buttress the integrity of the medical device validation process.
Design Controls in Medical Device Validation
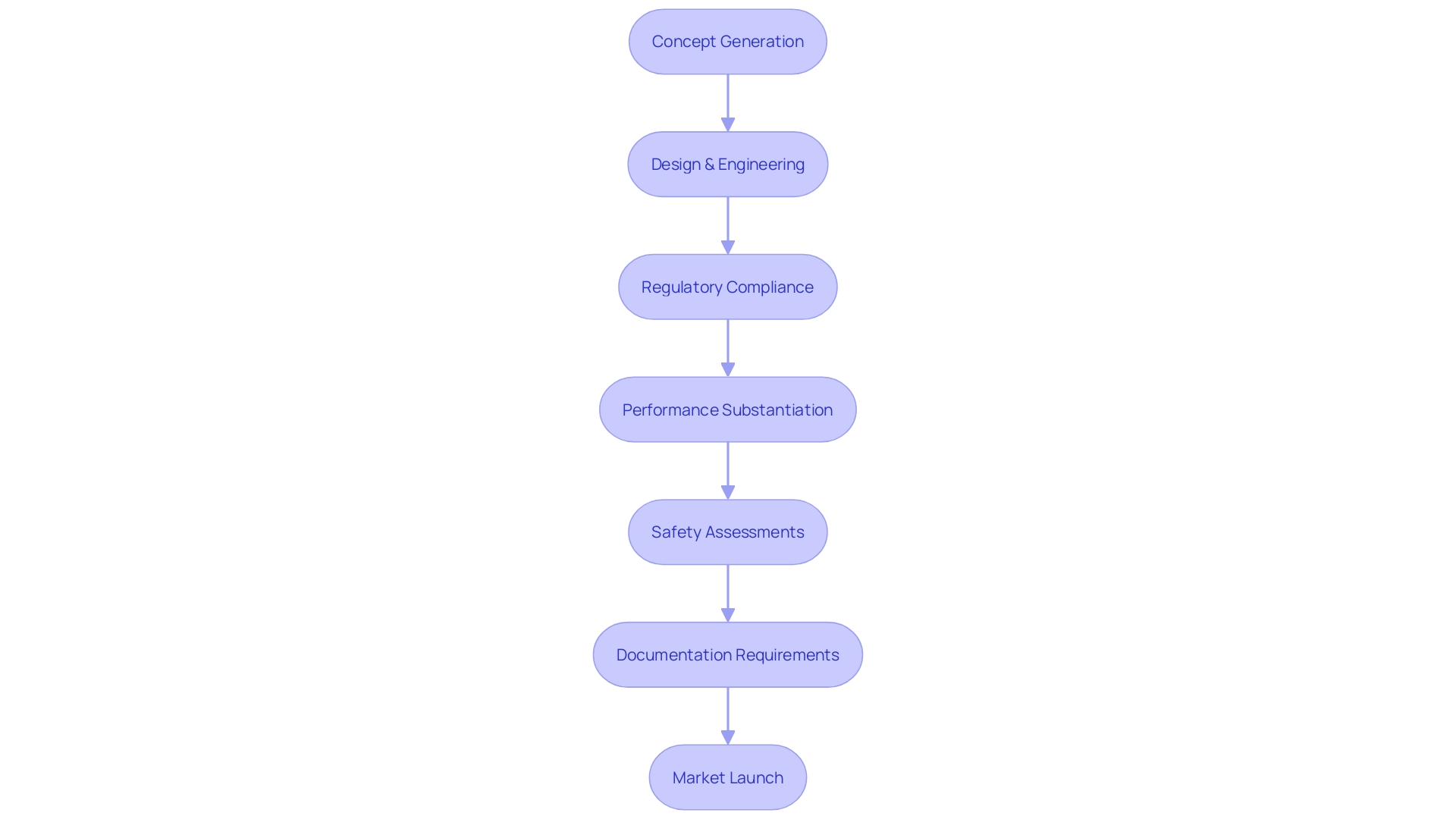
To ensure medical devices not only captivate with their innovation but also meet stringent regulatory requirements, a robust design control process is critical. This encompasses more than just the appealing 'shiny' aspects—those features that make a device stand out visually or technologically. Instead, it necessitates a meticulous adherence to procedural rigor throughout the entire development lifecycle, from design planning to input and output procedures, verification and validation activities, right through to design transfer and the management of changes.
It's about making sure all parts of the product, particularly software, can be thoroughly tested in a 'Design for Testability' approach, resulting in a device that not only looks and functions impressively but also meets all operational and regulatory expectations.
According to Mary Joyce, vice-president and general manager at UL Solutions mobility and critical systems group, the commitment to excellence in design control is exemplified by Michigan's thriving medical device sector. Their testing laboratories advance the safety and interoperability of devices, underpinning the vital importance of a well-executed design control process that addresses risks such as quality, safety, and cybersecurity.
Moreover, understanding the regulations governing the device is essential. As some obligations vary by region and are influenced by an array of policies, including the OECD's Conflict Minerals policy, it is prudent for manufacturers to aim for global regulatory compliance. This ensures that regardless of where the device ends up, it adheres to the most stringent of standards.
Companies that are part of the electronics supply chain, especially those dealing with Printed Circuit Board Assemblies, are likely conversant with directives like RoHS, highlighting the importance of regulatory knowledge across all stages of device manufacturing.
Finally, thorough knowledge of the device's use environment is crucial. Devices must be designed with the user in mind, taking into account where they will be stored, used, or disposed of. The environment's characteristics, such as lighting, can impact device interaction, emphasizing the need for user-centric design and, perhaps, ethnographic research to ensure the most effective and safe use of the medical technology.
This comprehensive approach to design control can be decisive for the successful introduction of a product to the market and its eventual acceptance by healthcare professionals and consumers alike.
Clinical Evaluation and Performance Monitoring
Gathering clinical data to evaluate the safety and efficacy of medical devices is a cornerstone in the validation process. It encompasses not only clinical trial outcomes but also expansive post-market surveillance endeavors and comprehensive literature reviews to acquire a multifaceted understanding of a device's impact in practical use. A pivotal tool used in capturing such data is Clinical Outcome Assessments (Coas), which collect evidence based on patient, clinician, and non-clinical observations, as well as performance-based results.
COAs are meticulously structured to include defined administration methods, data collection standards, and specific scoring and result interpretation techniques. One of the primary COA categories is Patient-Reported Outcomes (Pros) – these are unmediated reports from patients themselves that provide subjective data on health conditions, which may include symptoms and quality of life considerations. The collection of Pros is integral for understanding aspects that are otherwise unobservable, exemplified by assessments like the Exacerbations of Chronic Pulmonary Disease Tool – Patient Reported Outcome (EXACCT-PRO).
Additionally, manufacturers must systematically monitor and analyze medical device performance post-marketing to ensure sustained safety and functionality. This demands a robust infrastructure for adverse event reporting and ongoing studies, which in turn contributes essential data points to a device's lifecycle analysis. Incidents must be detailed, noting device type, any defects or malfunctions, and concurrent therapies that might influence outcomes, ultimately ensuring that devices remain reliable throughout their use.
Furthermore, the development of Digital Health Technologies (DHTs) is burgeoning, with the goal of achieving consumer-level usability while necessitating the same, if not increased, rigor in development and validation as traditional medical devices. A consensus from industry leaders underscores the importance of validating DHTs in their intended context while adhering to high standards of data quality, influenced by the same principles guiding software as medical devices (SaMD).
In the regulatory domain, the construction of a Clinical Evaluation Report (CER) for medical devices is a keystone in demonstrating conformance with international guidelines, particularly within the European Union for CE marking. As such, CER consultants have become pivotal in aiding manufacturers through these multifaceted evaluations – which form an essential element of medical device technical documentation.
In conclusion, clinical evaluation and performance monitoring underscore a device's ongoing validation and are pivotal in ensuring that medical technology advancements offer measurable, real-world benefits while adhering to stringent regulatory standards.
Regulatory Requirements for Medical Device Validation
Achieving regulatory compliance is pivotal for medical device manufacturers to ensure patient safety and gain market authorization. Compliance is more than just adherence to testing protocols; it involves a comprehensive understanding of various types of Clinical Outcome Assessments (Coas) such as Patient-reported Outcome (PRO) measures, which offer a direct report from patients on their health condition without external influence, providing unique insights into unobservable aspects like pain severity and life quality. In addition, adopting voluntary consensus standards developed by Standards Development Organizations (SDOs) is crucial.
Aligning with these standards—characterized by transparency, stakeholder involvement, and due process—enhances regulatory quality and facilitates innovation, as detailed in OMB Circular A-119 and ANSI Essential Requirements. These standards underpin a robust regulatory framework, bringing the necessary rigor to conformity assessments through activities like sampling, testing, and certification, ensuring that medical devices meet rigorous safety and performance criteria.
Furthermore, computational models employed in regulatory submissions require a precise definition of usage and an accompanying risk assessment. By establishing a clear Context of Use (COU) and addressing pertinent regulatory questions, manufacturers can integrate the model's role comprehensively. Recent developments, such as the expansion of testing capabilities at UL Solutions' Rochester Hills laboratory, exemplify the industry's response to escalating demand for medical device testing.
This facility allows for versatile testing methodologies and helps expedite the validation process while also focusing on reducing environmental contaminants like volatile organic compounds (VOCs). Industry professionals also emphasize the necessity for medtech companies to modernize compliance programs, warning against the inertia of manual processes which could otherwise hinder innovation.
Compliance is not static; it dynamically evolves with technology. As expressed by industry executives and authorities like the FDA, staying within regulatory boundaries while embracing smarter operational methods leads to faster market access. This agility in process adaptation is essential for embracing opportunities that ultimately benefit patient outcomes.
Consensus and conformity to high-quality standards are foundational to obtaining the confidence of regulators, partners, and patients, aligning all stakeholders with a uniform language and proactive approach to device performance and patient safety issues.
Benefits of Proper Medical Device Validation
Medical device validation stands as a cornerstone of healthcare innovation, merging regulatory compliance with patient safety and market presence. When medical device manufacturers substantiate the performance, safety, and reliability of their products through clear and defined validation procedures, they not only satisfy regulatory benchmarks but also fortify the device's reputation in the competitive healthcare market. Stringent regulatory oversight, such as the FDA's classification system, demands precise documentation and evidence of medical device efficacy, especially for high-risk Class III devices pivotal to sustaining life.
Reports and certifications based on patient-reported outcomes (Pros) and other clinical assessments exemplify thorough validation practices—these not only measure device performance but also enhance the transparency and understanding of their function.
Considering the medical devices industry's vast expanse of tools ranging from basic spectacles to intricate MRI machines, each device's validation is essential to ensure it functions safely within its healthcare context. Furthermore, the projected doubling of the 3D printing medical market by 2026, as reported by Global Data, underscores the imminent innovation and the imperative to maintain rigorous validation processes. This growth beckons an era where swift, precise technologies like CardiAQ's AI-assisted sensors for cardiac diagnostics will become more prevalent.
For such sophisticated devices, validity is paramount to gain a foothold in the market.
The thought is similar to product certifications in other industries—while they may seem arduous and reserved for large corporations, certification bodies have proven that they are attainable even for smaller companies focused on innovation. By adhering to a 'best practice manual,' companies implement standards that enable proactive handling of device performance issues, enhancing patient safety. The GlobalData report reflects an optimistic future wherein companies large and small may leverage validations as both a guiding light for internal processes and as a beacon of trust and quality for consumers.
Challenges in Medical Device Validation
The landscape of medical device development is characterized by its diversity and complexity, with an array of instruments that cater to various healthcare needs, from simple tools like spectacles to sophisticated systems such as MRI machines and pacemakers. Each device demands meticulous consideration of design and regulatory requirements to ensure patient safety and efficacy.
Pioneering medical device testing in specialized laboratories, such as the UL Solutions facility in Rochester Hills, Michigan, is part of an industry-wide response to the pressing need for more robust evaluation protocols. This forward-thinking approach aims to mitigate risks and uphold a high standard of device quality, delving deep into areas like safety, security, usability, and interoperability.
Lessons from The Bleeding Edge documentary reveal crucial insights into the FDA's 510(k) clearance process and underscore the imperative nature of extensive testing, especially in light of past episodes where devices led to adverse outcomes such as cobalt poisoning or organ puncture. The central message here is that the route to market for medical devices must be navigated with utmost diligence, prioritizing patient well-being above all.
Moreover, the reflection on 'technosolutionism' from the RIH framework stresses the importance of shifting the discourse from race to racism in health outcomes, thus casting a critical eye on how medical technology intersects with social issues.
Research design is paramount in the creation pathway, starting with a clear, well-defined question that underpins the structure of the entire study. Detailed planning in the experimental layout helps reduce biases and confounding effects, positioning controlled studies as indispensable components that contrast treatment and control groups for impactful insights.
Embracing an integrated approach, manufacturers ought to seek development partners who not only possess technical knowledge but also profound regulatory insight and market savviness. These partnerships enhance overall project success through seamless phase transitions, conserving resources and optimizing timelines.
Finally, the very inception of a medical device project can determine its trajectory. Ensuring the initial team meeting establishes priorities and delineates responsibilities paves the way for a harmonious effort, uniting stakeholders behind a shared vision and paving the way for a successful, patient-centric end result.
Conclusion
In conclusion, medical device validation is a critical process that ensures the safety, efficiency, and reliability of healthcare technology. It involves rigorous testing, evaluation, and documentation, covering a wide range of devices with unique challenges related to human and technological diversity.
Clinical Outcome Assessments (COAs) play a vital role in capturing unobservable aspects like pain and life quality, while postmarket reporting helps identify defects or malfunctions. Thorough validation processes and compliance programs must adapt to maintain stringent safety and quality standards in an industry of rapid innovation.
Michigan's UL Solutions laboratory exemplifies the focus on safety, security, usability, and interoperability. By adhering to regulatory requirements and implementing a robust design control process, medical device manufacturers can gain market authorization and build a strong reputation in the competitive healthcare market.
Despite challenges such as the diverse nature of medical devices and the need for extensive testing, validation is essential in ensuring device effectiveness and reliability. It safeguards patient health, mitigates risks, and ensures excellence in product launches.
In summary, medical device validation plays a crucial role in safeguarding patient health and safety, mitigating risks, and ensuring excellence in product launches. Compliance with regulatory standards and a robust design control process are essential. By embracing innovation and remaining dedicated to adherence and testing, medical device manufacturers can bring safe and effective products to market and uphold their commitment to patient care.




