Introduction
The regulatory landscape for post-market clinical follow-up studies in Latin America is a complex and ever-evolving field. Each country in the region has its own protocols and mandates, making it essential for researchers to carefully review and adhere to regulatory requirements. The importance of robust post-market scrutiny of healthcare solutions is exemplified by the mental health burdens faced by low- and middle-income countries in Latin America.
In this article, we will explore the process of developing study protocols for post-market clinical follow-up studies, participant recruitment and informed consent, data collection and analysis, and the reporting and dissemination of study findings. We will also emphasize the need to prioritize ethical considerations and patient safety throughout the entire post-market phase. By understanding and adhering to the regulatory landscape, researchers and study sponsors can ensure the ongoing safety and efficacy of medical devices in Latin America.
Understanding the Regulatory Landscape for Post-Market Clinical Follow-Up Studies
Comprehending the regimen for post-market clinical follow-up (PMCF) studies in Latin America is imperative for safeguarding the welfare of patients using medical devices. Every nation in the region possesses distinct protocols and mandates concerning PMCF, which calls for meticulous review and adherence to guarantee not only regulatory conformity but also the ethical execution of such studies.
For instance, Vida Plena’s pioneering work confronting mental health challenges demonstrates the scale and complexity of addressing significant health burdens in Latin America, where up to 80% of the mental health disease burden falls upon low- and middle-income countries, accentuating the necessity for rigorous post-market scrutiny of healthcare solutions.
Medical devices undergo a rigorous examination in the post-market phase to continue evaluating their effectiveness and safety. This includes leveraging various data collection methods from electronic health records to active surveillance through registries or studies. The data gathered provide critical insights, which are quintessential for the continuous enhancement of medical device performance.
Recent developments in Colombia's healthcare regulation field, such as the expected appointment of a new head of INVIMA, illustrate the dynamic nature of regulatory oversight in Latin America. Meanwhile, ANVISA's digitization efforts to improve access to medication information epitomize the innovative strides in enhancing drug safety and public health strategies.
Amidst this backdrop, Honduras’s ARSA agency reports a remarkable 60% increment in inspection activities and a commitment to bolster pharmacovigilance training in 2023, signifying a heightened focus on medical device surveillance and patient safety. The implementation of new licenses and technical centers further underscores the progress made in post-market oversight.
As the global arena recognizes the need to adopt best practices in decentralized clinical trials and digital vigilance, it is clear that the medical community in Latin America must also prioritize robust PMCF studies to maintain the highest standards of patient care and safety in the region.
Developing a Study Protocol for Post-Market Clinical Follow-Up Studies
The success of post-market clinical follow-up (PMCF) studies hinges on a meticulously crafted protocol, delineating the objectives, methodologies, and strategies for data acquisition. The underpinning of an insightful PMCF lies in its protocol design—it should articulate the study endpoints, duration, and a sample size robust enough to yield statistically valid outcomes that are relevant for patient safety and device efficacy.
Ethical considerations and participant criteria, whether inclusionary or exclusionary, must be detailed, ensuring the research is carefully tailored to capture the precise demographic and clinical conditions relevant to the device's real-world application. This vigilance in protocol design is further emphasized by the obligation to report any device-related adverse events, use errors, or product issues—critical for maintaining the integrity of the data collected.
Alongside these study fundamentals, researchers must address the dynamic nature of medical device technologies—a facet that includes but is not limited to market incentives, intellectual property concerns, and the ever-evolving global regulatory landscape. The case studies in health and medicine suggest that this multifaceted approach is imperative, offering not only historical context but also ethical, legal, and social perspectives pertinent to the governance of such technologies.
The World Health Organization's definition of medical devices underscores the sheer variety and complexity of these technologies, ranging from the mundane, such as tongue depressors, to the intricate, such as diagnostic software and prostheses. Consequently, the study protocol must be sufficiently adaptable to encompass a wide array of devices, each with unique implications for patient care.
As emerging technologies continue to develop and intertwine with the healthcare landscape, the necessity for post-market data becomes clear. This data must speak not only to a device's safety and effectiveness but also to its coverage and integration within healthcare systems. The FDA's role in the U.S., juxtaposed with decision-making bodies like CMS and private health plans, illustrates the multi-stakeholder environment within which PMCF studies operate.
Feedback loops from PMCF studies are instrumental in shaping the trajectory of medical devices—informing manufacturers, regulatory agencies like the FDA, and payors about safety concerns, operational malfunctions, and overall patient outcomes. These studies, when executed with rigor and a holistic view of the medical device ecosystem, ensure that the device in question continues to meet the highest standards of patient care post-commercialization.
Participant Recruitment and Informed Consent
The successful engagement of participants for post-market clinical follow-up studies hinges on a detailed and compassionate approach that reflects the demographics and conditions of patients who will ultimately utilize the medical device. The informed consent process is at the heart of ethical clinical research and entails a comprehensive briefing for each participant on the study's aim, the risks and benefits involved, and the privacy safeguards in place. Participants must understand that their involvement is voluntary and free from any undue pressure.
Actively addressing patient concerns, such as the time required for participation and study procedure complexities, is essential to boost enrollment and retention rates — as suggested by the recruitment challenges within chronic kidney disease research. Lessons from patient experiences, such as Barbara's unexpected cardiac diagnosis resulting from study participation, reinforce the benefits of research involvement, from improved personal health insights to potentially life-saving interventions. Empowering patients with knowledge, the right online platforms, such as The New Normal, have demonstrated efficacy in bridging the gap between research opportunities and community awareness.
Studies have indicated that patient trust in healthcare providers, the aspiration to aid future medical treatment, and the desire to contribute to medical research are powerful motivators. This altruism is particularly prominent among patients with specific conditions hoping to improve their and their families' health outcomes.
Furthermore, aligning trial participation with ethical standards means ensuring that the sacrifices participants make, such as time and potential risks, are recognized and fairly compensated. This sets a standard of fairness and equity, often compared to the compensation of other roles in society, such as first responders, who also serve the public good.
In light of these factors, protecting the rights and concerns of clinical study participants within Latin America is not just a regulatory requirement but a foundational practice that promotes the safety, efficacy, and ethical conduct of post-market clinical follow-up studies.
Data Collection and Analysis
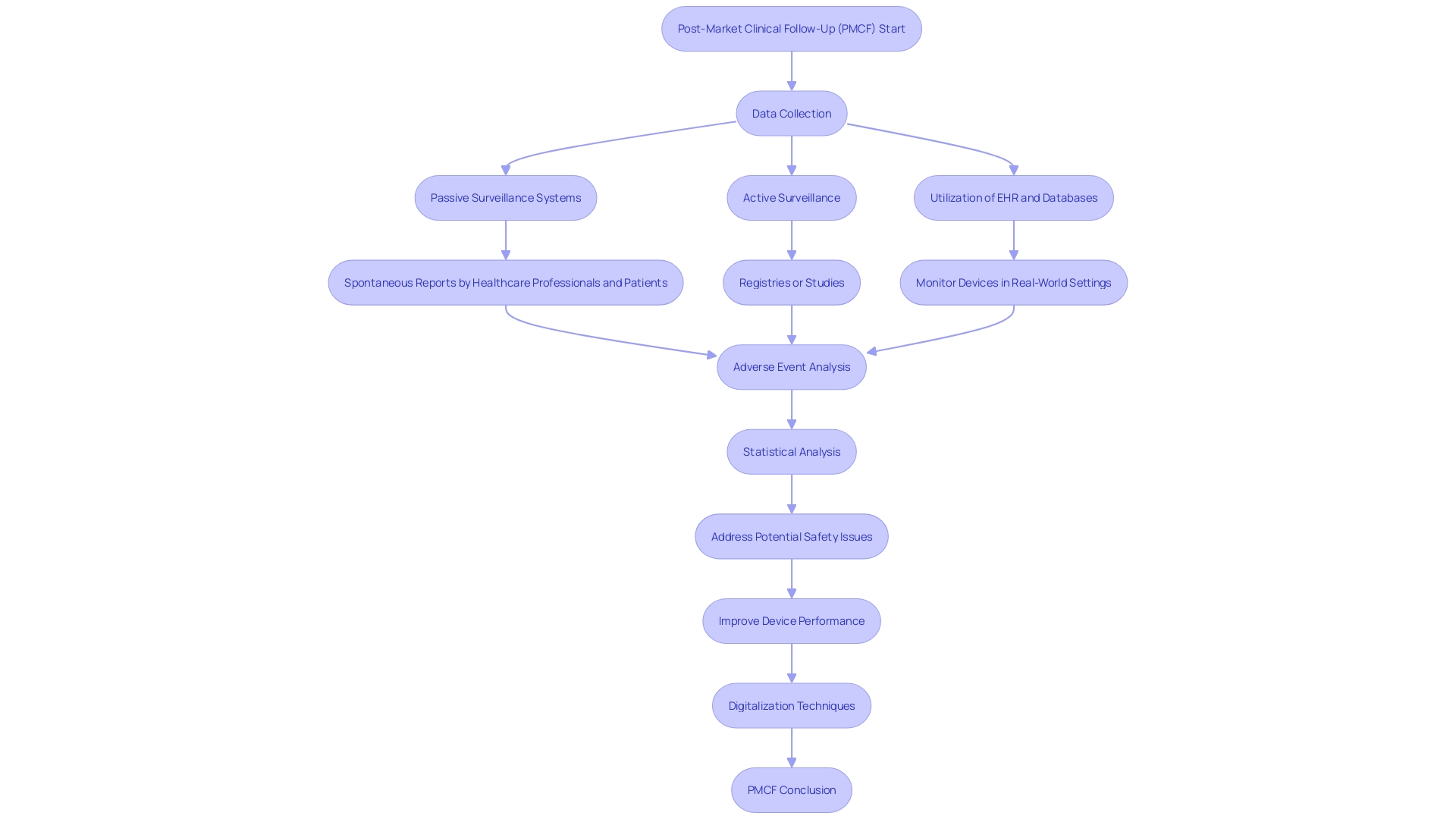
The landscape of post-market clinical follow-up (PMCF) studies in Latin America has evolved into a key phase for ensuring medical devices' safety and effectiveness beyond their initial market approval. These studies provide a systematic framework for monitoring devices once they are widely in use, utilizing various data streams to capture an accurate picture of device performance in real-world scenarios. High-quality data collection methods are critical, and can include examining patient medical records, leveraging electronic health records, factoring in patient-reported outcomes, and scrutinizing adverse event reports.
With a vigilant eye on complex medical devices, it is crucial to dissect and record all aspects of any adverse event including device type, manufacturer details, brand, and, if possible, batch number. Understanding the nature of the incident is key; whether it was a device malfunction, defect or user error is vital information. Knowledge of who was operating the device at the incident's occurrence and the presence of any concurrent therapies that might have influenced the event bolster the robustness of collected data.
Diving into this data with refined statistical approaches enables researchers to surface actionable insights—shaping decisions that enhance patient safety and treatment efficacy. The successful interpretation of PMCF data hinges on maintaining stringent standards for data integrity and participant privacy, underlining the high stakes of this work.
Utilizing novel digitalization techniques also plays a significant role in PMCF studies by streamlining data acquisition and analysis processes, allowing for intelligent and efficient study designs. Advanced methodologies, including artificial intelligence, enhance interpretation of diverse data sets from lab results to patient diaries, magnifying the potential for profound insights.
Recent demonstrations of robust PMCF frameworks include the utilization of SmartHeart technology—showcased at important industry meetings—which avails of modern connectivity via Bluetooth to relay ECG results to healthcare professionals, embodying the advancement in remote patient monitoring technologies. As per industry reports, the market for such remote monitoring technologies is burgeoning, projected to reach significant financial valuations by the end of this decade.
In the current climate of clinical research, where there's an abundance of data but also an imperative to extract meaning, it's evident that a comprehensive data strategy laid out before protocol design can dictate the efficacy of the PMCF. Such preemptive planning can ensure that the ever-increasing volumes of clinical data are funneled into insights that have material impacts on patient outcomes, and ultimately, global health.
Reporting and Dissemination of Study Findings
Completing comprehensive analysis of post-market data is not the endpoint but a pivotal moment for ensuring the continuous safety and efficacy of medical devices in Latin America. A meticulous compilation of the study's core elements—objectives, methodology, results, and extensive discussions—is essential. This allows an in-depth representation of the data, encompassing both its strengths and any influencing limitations.
The critical practice of knowledge dissemination takes various forms, from peer-reviewed journal publications to presentations at scientific gatherings. Additionally, it encompasses transparent communications with entities such as regulatory authorities and all relevant stakeholders.
Such transparency is aligned with the understanding that while forecasts about business strategies and product outcomes are insightful, they inherently carry uncertainties. Readers are hence guided to interpret forward-looking statements with caution, acknowledging that they do not serve as infallible predictors.
Primarily, detailed reporting addresses device issues—whether defects or malfunctions—and actions taken during adverse events. It also incorporates the necessary logic and comprehensibility that foster explainability, guaranteeing that the information meets the needs of its intended users—healthcare professionals, patients, and stakeholders. This approach eschews opacity in favor of clarity, which is fundamental not only for patient safety but also for maintaining robust post-market surveillance (PMS), which indefinitely extends the window for monitoring medical device performance in the real-world healthcare environment.
To that end, the concepts of logic and explainability reinforce the idea that transparency isn't just an option, but a requisite for trust and efficacy in the post-market phase. It demands thoughtful communication strategies, ensuring that pivotal data, risks, and clinical outcomes are conveyed effectively and aligned with users' understanding and the healthcare context. Considering that medical devices range from basic items such as gloves to advanced diagnostic machines, the post-market phase is significantly crucial to address the dynamic nature of device applications and their integration within the health care landscape.
Ensuring Ethical Considerations and Patient Safety
Ensuring the ongoing safety and efficacy of medical devices through post-market clinical follow-up (PMCF) studies is a dynamic and multifaceted endeavor. It is critical for researchers and study sponsors to navigate this complexity with a firm commitment to ethical practices and patient safety. Establishing appropriate measures to protect the privacy of study participants and the responsible handling of informed consent is central to this commitment.
Stringent safety monitoring procedures are also crucial to promptly identify and address unforeseen safety concerns, thereby safeguarding the integrity of the study and the well-being of participants.
Moreover, the management of PMCF studies must take into consideration a spectrum of factors that influence the evolution of emerging medical technologies. These include market incentives, intellectual property rights, and the broader governance framework within which these studies operate. Vigilant adherence to ethical guidelines and regulatory requirements, such as those set forth by the FDA, ensures not only the safety of participants but also fortifies the credibility and acceptance of study findings.
For instance, the FDA mandates that the presentation of drug risks in direct-to-consumer advertisements be clear, conspicuous, and neutral, highlighting the agency's emphasis on informed consumer understanding.
The far-reaching implications of adeptly conducted PMCF studies are underscored by personal accounts of clinical trial participants, many of whom take on the potential risks of research with an altruistic vision toward future generations. Their experiences — dealing with disease, enduring invasive tests, and grappling with complex treatments — are emblematic of the grave responsibilities shouldered by clinical researchers. The insights gained through case studies and personal testimonials underscore the urgency for establishing a cross-sectoral governance framework for emerging health technologies that not only addresses ethical, legal, and social issues but also aligns with clinical research integrity.
Reflecting on the stark realities of clinical trials, it is incumbent upon the research community to effectively document all aspects of device performance and patient interactions. Detailed reporting of device-related problems, such as defects or malfunctions, and the situational context of adverse events provides a critical feedback loop for identifying product issues and enhancing patient care strategies. Empirical data on device type, manufacturer, brand name, and associated adverse events offer a granular understanding of the real-world impact of medical devices post-market, contributing to the continuous improvement in the safety profile of these essential health tools.
In conclusion, the pursuit of advancing medical science through PMCF studies in Latin America and beyond demands a conscientious synergy of ethical practice, regulatory compliance, and empathetic engagement with patients. It is a pursuit built on trust — trust that is earned through relentless dedication to safeguarding the rights and well-being of participants and upholding the highest standards of clinical research.
Conclusion
In conclusion, the regulatory landscape for post-market clinical follow-up (PMCF) studies in Latin America is complex and requires adherence to country-specific protocols. Robust PMCF studies are essential for addressing mental health burdens in low- and middle-income countries. Well-designed study protocols outline objectives and methodologies, while ethical considerations and participant criteria ensure relevancy.
Data collection methods, including electronic health records and patient-reported outcomes, require stringent standards for integrity and privacy. Novel digitalization techniques, such as artificial intelligence, enhance data interpretation for valuable insights.
Transparent reporting and dissemination of study findings, along with open communication with regulatory authorities, build trust and meet the needs of healthcare professionals and patients. Ethical practices and patient safety must remain a priority throughout the PMCF process, and adherence to guidelines and requirements ensures credibility. Detailed reporting of device-related issues and adverse events provides feedback for improving patient care.
In conclusion, advancing medical science in Latin America through PMCF studies relies on ethical practices, regulatory compliance, and empathetic patient engagement. By prioritizing patient safety and upholding high standards of research, medical devices' safety and efficacy can be ensured, ultimately improving patient care.




