Overview
The article examines the ethical considerations pertinent to clinical trials in Chile, asserting the critical nature of informed consent, respect for autonomy, beneficence, non-maleficence, and justice. It delineates the challenges faced, including:
- Variability in the application of ethical guidelines
- Cultural perceptions
- Logistical hurdles
Furthermore, it underscores the imperative for comprehensive training and stringent regulatory oversight to safeguard participant rights and ensure ethical compliance in research practices.
Introduction
In the complex landscape of clinical trials, ethical considerations stand as the cornerstone of responsible research practices. With the rights and well-being of participants at stake, principles such as:
- Informed consent
- Respect for autonomy
- Beneficence
- Non-maleficence
- Justice
become paramount. Researchers navigating these ethical mandates face significant challenges, including inconsistent understanding of guidelines and cultural barriers that may impact participant engagement. In Chile, a robust regulatory framework aims to uphold these ethical standards; however, hurdles remain in ensuring compliance and fostering trust among participants. This article delves into the critical ethical dimensions of clinical trials, examining both the regulatory environment and the challenges in achieving ethical compliance while highlighting the importance of informed consent and effective patient recruitment strategies.
Define Ethical Considerations in Clinical Trials
Ethical considerations for trials in Chile serve as foundational principles designed to safeguard the rights, safety, and welfare of individuals involved. Essential principles include knowledgeable consent, respect for autonomy, beneficence, non-maleficence, and justice.
- Awareness consent is paramount, ensuring participants are fully informed about the risks and benefits associated with their involvement in a study. This is especially critical, as recent findings indicate that a limited number of researchers reported receiving formal training on the necessity of obtaining consent, often relying on experience or self-education. This gap underscores the urgent need for enhanced training and resources to improve the understanding and execution of consent practices.
- Respect for autonomy emphasizes the importance of empowering individuals to make informed decisions regarding their participation.
- The ethical considerations for trials in Chile highlight the significance of beneficence and non-maleficence, urging researchers to maximize potential benefits while minimizing harm to participants.
- Justice ensures the equitable selection of participants, reinforcing the ethical considerations for trials in Chile and preventing any group from being unfairly burdened or excluded from the benefits of research.
As the moral landscape evolves, ongoing dialogue and research are essential. Real-world examples illustrate the significance of these principles in practice, reinforcing the necessity for strict moral standards in clinical trials. For instance, a case study titled "Training and Guidelines for Consent" demonstrated the need for improved training and resources to enhance researchers' comprehension and execution of consent practices.
The commitment to ethical considerations for trials in Chile is crucial for preserving the integrity of clinical research and fostering public trust in the medical field. As one researcher noted, "I get a gut feeling" about the challenges faced in ensuring informed consent, highlighting the subjective nature of these experiences. This reinforces the importance of organized training and guidelines in navigating moral complexities.
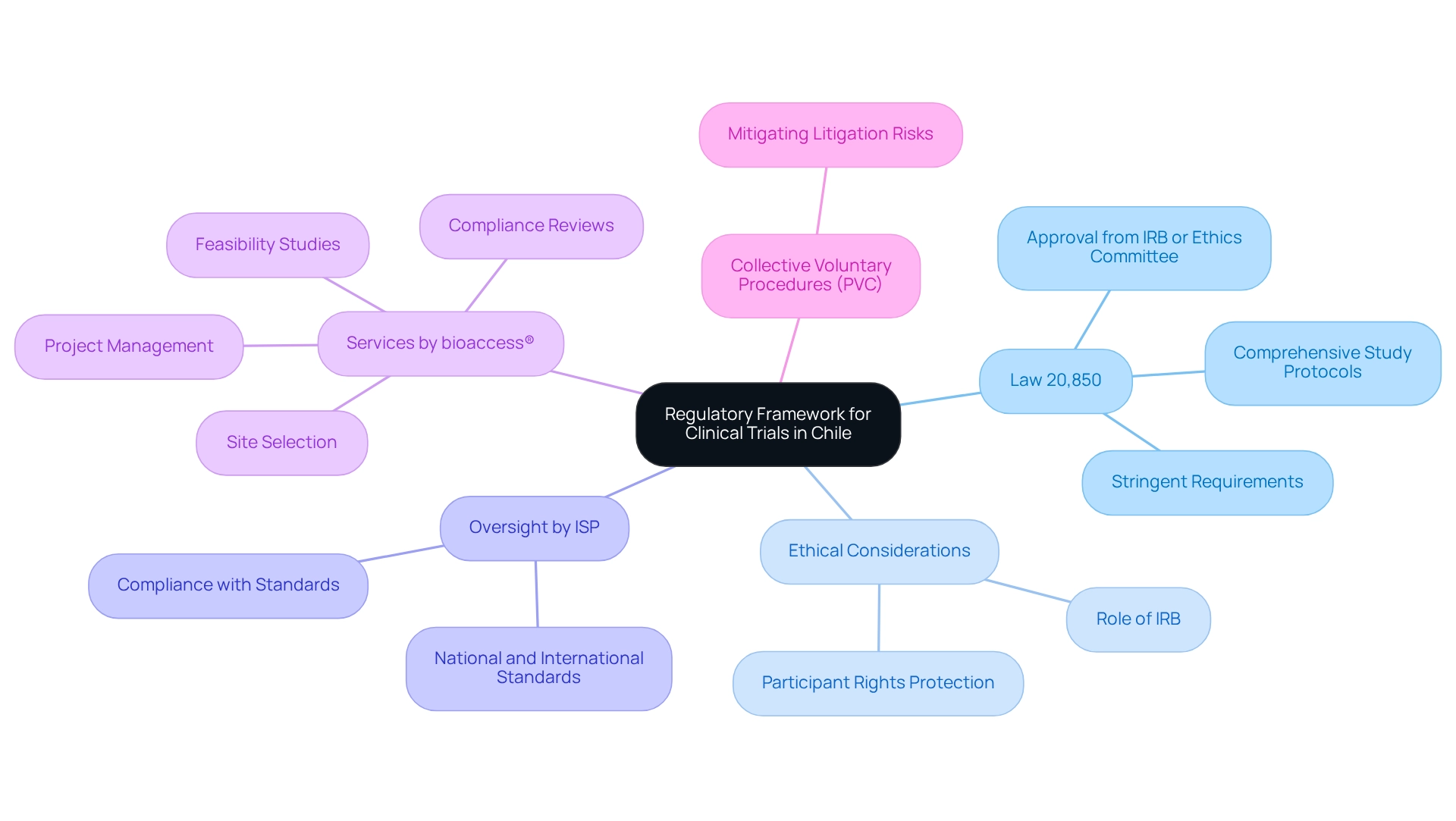
Explore the Regulatory Framework for Clinical Trials in Chile
In Chile, the regulatory framework governing clinical studies is anchored by Law 20,850, which imposes stringent requirements for conducting clinical research. This law mandates that all clinical trials address ethical considerations by securing approval from an Institutional Review Board (IRB) or Ethics Committee, tasked with evaluating the moral implications of proposed research. The Instituto de Salud Pública (ISP) oversees this regulatory process, ensuring compliance with both national and international ethical standards. Researchers are required to submit comprehensive study protocols detailing methodology, subject recruitment strategies, and informed consent processes. Such rigorous regulatory oversight is vital for protecting participant rights and embedding ethical considerations throughout the research process in Chile.
Furthermore, bioaccess® offers an extensive suite of clinical study management services, including:
- Feasibility studies
- Site selection
- Compliance reviews
- Setup
- Import permits
- Project management
- Reporting on serious and non-serious adverse events
These services are essential for navigating the complexities of the regulatory landscape. The Collective Voluntary Procedures (PVC) established by the CRPA provide companies with strategies to mitigate litigation risks during recalls, underscoring the significance of compliance within this regulatory framework. As a result, Chile has emerged as a leader in medical device clinical studies in Latin America, highlighting its commitment to ethical considerations and the enhancement of healthcare quality and moral standards.
Identify Challenges in Achieving Ethical Compliance
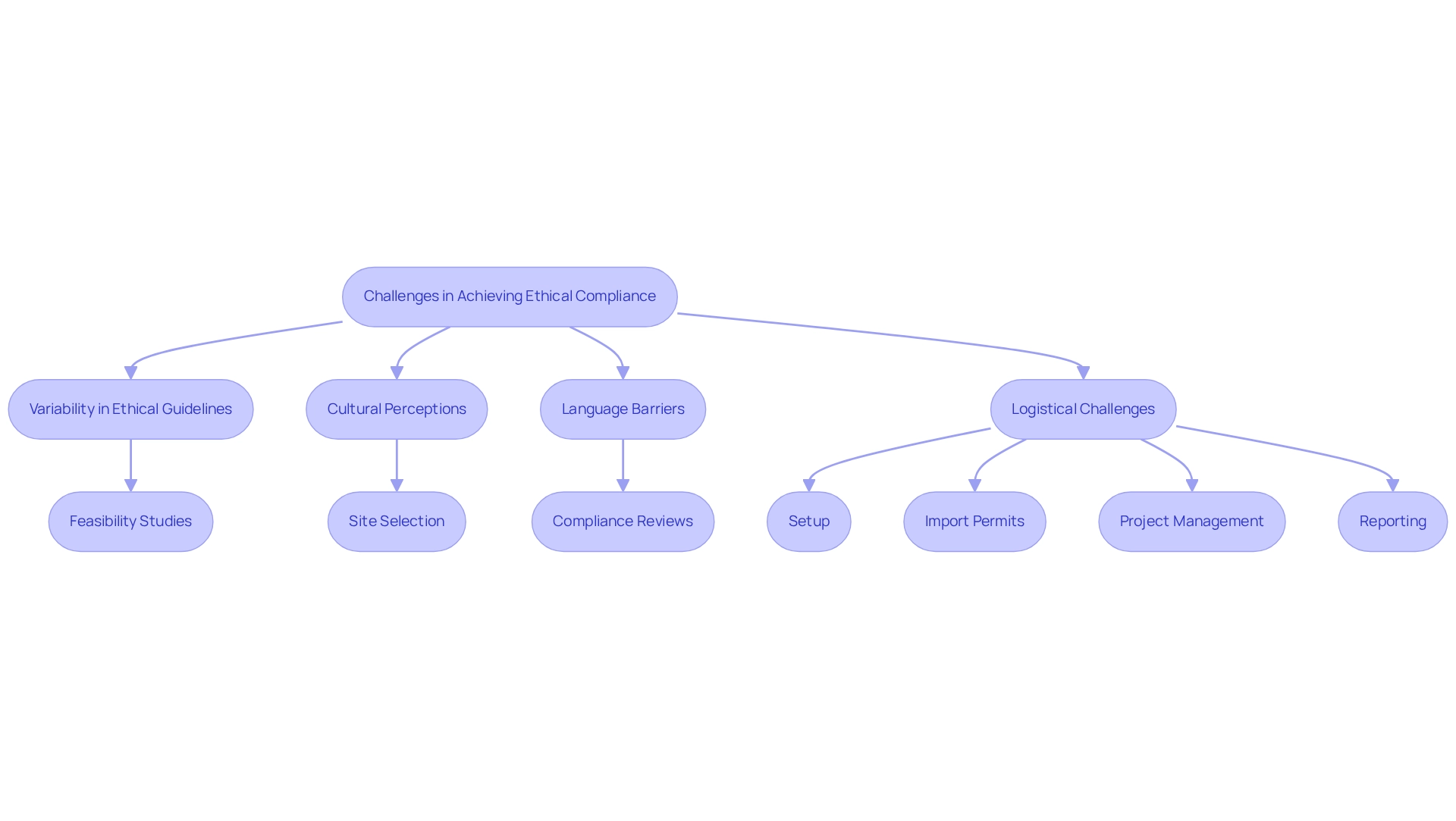
Attaining ethical considerations for trials in Chile presents significant obstacles that demand attention. A critical issue lies in the variability of understanding and application of ethical guidelines among researchers and institutions, which can lead to inconsistencies in acquiring consent and honoring the rights of participants. Additionally, cultural elements may shape individuals' perceptions of clinical studies, potentially affecting their willingness to engage in research. Language barriers and varying levels of health literacy further complicate the informed consent process, making it difficult for individuals to fully comprehend the implications of their involvement.
Moreover, logistical challenges, such as delays in regulatory approvals and resource limitations, can hinder the timely implementation of studies, thereby impacting ethical adherence and participant safety. To effectively address these challenges, comprehensive clinical study management services provided by bioaccess are essential. These services include:
- Feasibility studies to assess site readiness
- Site selection to ensure suitable environments for experiments
- Compliance reviews to enhance consent processes
- Setup to facilitate initiation
- Import permits for investigational devices
- Project management for efficient execution
- Reporting to uphold transparency and accountability
Collectively, these services are vital for ensuring that assessments adhere to ethical considerations for trials in Chile and protect the rights of those involved. Collaboration among stakeholders is crucial for overcoming these challenges and fostering a research environment that prioritizes ethical standards and participant welfare.
Examine Patient Recruitment and Consent Issues
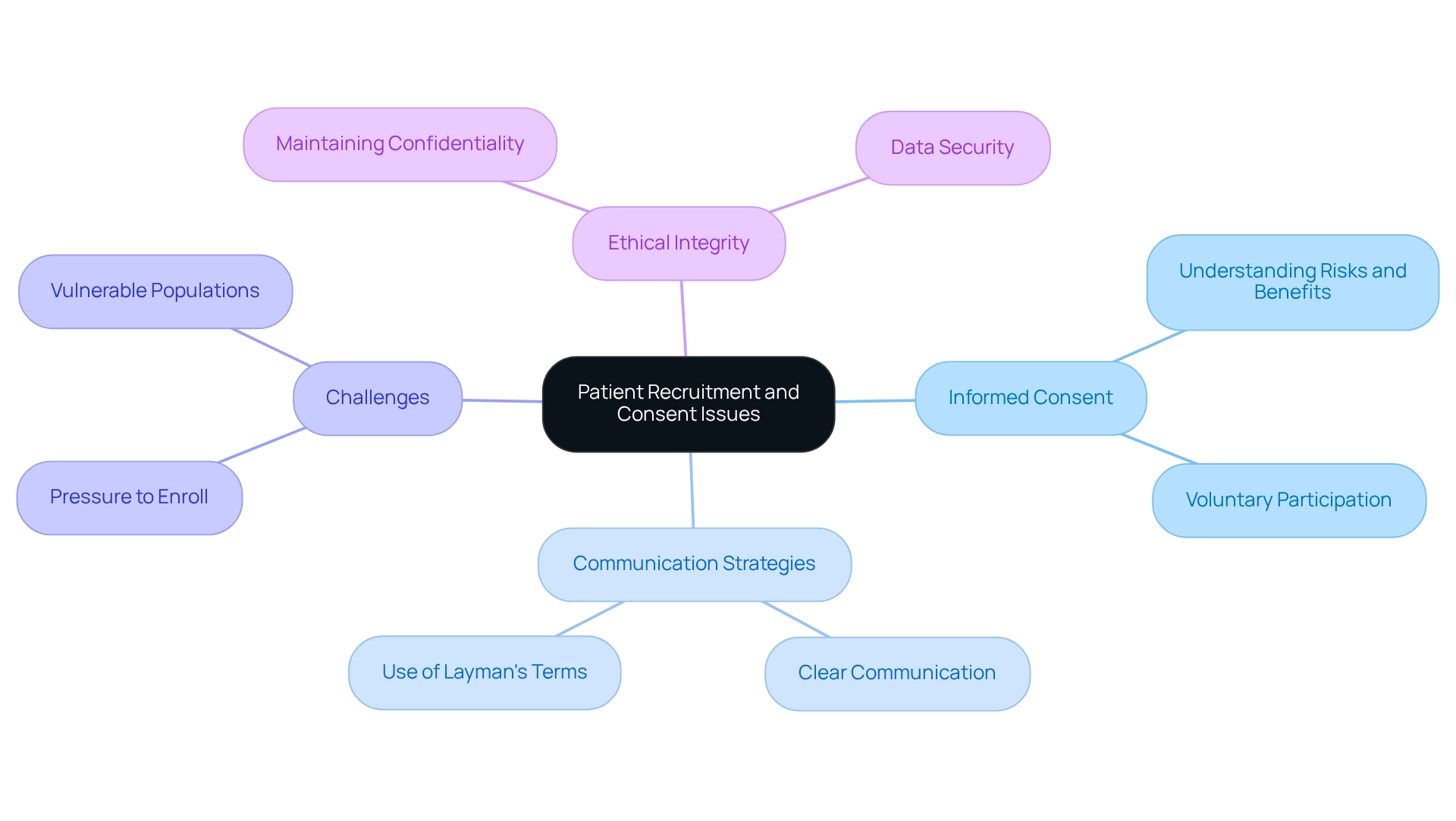
Participant recruitment and knowledgeable consent are essential elements of ethical clinical trials, particularly in Chile. Ethical considerations for trials necessitate that researchers ensure potential contributors are adequately informed about the study's purpose, procedures, risks, and benefits before obtaining their consent. This process demands clear communication, employing layman's terms to guarantee comprehension.
However, challenges often arise, such as individuals feeling pressured to enroll due to the influence of healthcare providers or societal expectations. Furthermore, vulnerable populations may encounter unique obstacles in the consent process, requiring tailored approaches to safeguard their rights. Researchers must also navigate the complexities of maintaining subject confidentiality and data security throughout the trial.
By addressing these issues, researchers can enhance the ethical integrity of their studies, taking into account the ethical considerations for trials in Chile, and foster trust among participants.
Conclusion
The ethical considerations surrounding clinical trials are paramount for safeguarding the rights and well-being of participants. Foundational principles such as informed consent, respect for autonomy, beneficence, non-maleficence, and justice underpin responsible research practices. There is a pressing need for enhanced training and resources for researchers, particularly in the realm of informed consent, which remains a critical area for improvement.
In Chile, the regulatory framework, spearheaded by Law 20,850, establishes rigorous standards for conducting clinical trials, ensuring that ethical considerations are embedded throughout the research process. The oversight provided by Institutional Review Boards and the Instituto de Salud Pública is vital for maintaining compliance with both national and international ethical norms. However, challenges persist, including variability in understanding ethical guidelines among researchers, cultural influences on participant perceptions, and logistical hurdles that can impede ethical compliance.
Moreover, patient recruitment and informed consent processes necessitate careful navigation to ensure that potential participants are fully informed and not unduly pressured. Addressing these challenges is crucial for fostering trust and ensuring that the integrity of clinical trials is upheld. By prioritizing ethical standards and enhancing communication strategies, researchers can cultivate a more transparent and respectful environment for participants.
Ultimately, the commitment to ethical compliance in clinical trials not only protects participants but also bolsters the overall credibility of clinical research. As the landscape of medical research continues to evolve, ongoing dialogue and a collective effort to uphold ethical integrity will be imperative for advancing clinical trials in a responsible manner.
Frequently Asked Questions
What are the foundational ethical principles for trials in Chile?
The foundational ethical principles for trials in Chile include knowledgeable consent, respect for autonomy, beneficence, non-maleficence, and justice.
What is knowledgeable consent and why is it important?
Knowledgeable consent ensures that participants are fully informed about the risks and benefits associated with their involvement in a study. It is important because many researchers lack formal training on obtaining consent, highlighting the need for better education and resources.
How does respect for autonomy play a role in clinical trials?
Respect for autonomy emphasizes empowering individuals to make informed decisions regarding their participation in clinical trials.
What do beneficence and non-maleficence mean in the context of clinical research?
Beneficence urges researchers to maximize potential benefits for participants, while non-maleficence emphasizes minimizing harm to participants.
What does the principle of justice ensure in clinical trials?
The principle of justice ensures equitable selection of participants, preventing any group from being unfairly burdened or excluded from the benefits of research.
Why is ongoing dialogue and research important in the context of ethical considerations for trials?
Ongoing dialogue and research are essential as the moral landscape evolves, helping to reinforce the necessity for strict moral standards in clinical trials.
Can you provide an example that illustrates the need for improved consent practices?
A case study titled 'Training and Guidelines for Consent' demonstrated the need for enhanced training and resources to improve researchers' comprehension and execution of consent practices.
What is the significance of ethical considerations for trials in preserving public trust?
Commitment to ethical considerations is crucial for preserving the integrity of clinical research and fostering public trust in the medical field.




