Introduction
Argentina's regulatory landscape for medical devices is meticulously structured by the National Administration of Drugs, Foods, and Medical Technologies (ANMAT), ensuring that all medical devices adhere to rigorous safety and efficacy standards. This comprehensive framework encompasses the registration, classification, and post-market surveillance of medical devices, thereby providing a robust system for regulatory compliance. The regulatory process involves a detailed risk assessment that classifies medical devices into different categories based on their intended use and associated risk levels, aligning with global trends and significantly influencing market pathways.
The harmonization of assessment criteria across countries further supports the evaluation of digital health solutions and fosters innovation. ANMAT’s regulatory oversight extends to post-market activities, crucial for addressing safety issues arising from real-world use, aligning with global regulatory trends and anticipated significant changes. This article delves into the intricacies of Argentina’s regulatory framework, the classification of medical devices, clinical trial requirements, and the current challenges and opportunities within this evolving landscape.
Regulatory Framework for Medical Devices in Argentina
Argentina's regulatory framework for health instruments is influenced by the National Administration of Drugs, Foods, and Medical Technologies (ANMAT), guaranteeing that all health instruments adhere to rigorous safety and effectiveness criteria. ANMAT's comprehensive guidelines cover the registration, classification, and post-market surveillance of health products, providing a robust framework for regulatory compliance.
The regulatory process involves a systematic evaluation of the risks associated with healthcare instruments, leading to their classification into different categories based on their intended use and risk levels. This method corresponds with worldwide patterns where the categorization of healthcare instruments is essential for identifying the suitable regulatory route. For instance, market pathways depend heavily on classification of products, as seen in the U.S. where the FDA requires businesses to register their establishments and list their items accordingly.
Furthermore, the importance of harmonizing assessment criteria across countries cannot be overstated. A harmonized approach ensures that digital solutions and mobile apps in the health sector are evaluated with uniform criteria, thereby supporting decision-making while fostering innovation and research. This is especially significant as the worldwide healthcare equipment sector encounters rising regulatory requirements, requiring effective strategies to adjust and prosper in these changing environments.
ANMAT’s regulatory oversight extends to post-market activities, which are essential for addressing any safety issues that arise from real-world use. Post-market reports, such as the FDA's 522 Studies and Post-Approval Studies (PAS), are instrumental in monitoring product performance and safety after market entry. This ongoing monitoring aids in upholding high standards of healthcare product safety and effectiveness, ensuring that producers can respond to any issues quickly.
The agency’s efforts are in line with global regulatory trends, where significant changes are anticipated in 2024. These include new regulations for laboratory-developed tests (LDTs) in the U.S., which highlight the dynamic nature of the regulatory environment. As such, manufacturers must stay abreast of these changes to ensure compliance and leverage opportunities for market entry.
In summary, ANMAT’s regulatory framework for healthcare products in Argentina is a model of thorough oversight, aligning with international best practices to ensure the safety and efficacy of healthcare items in the market.
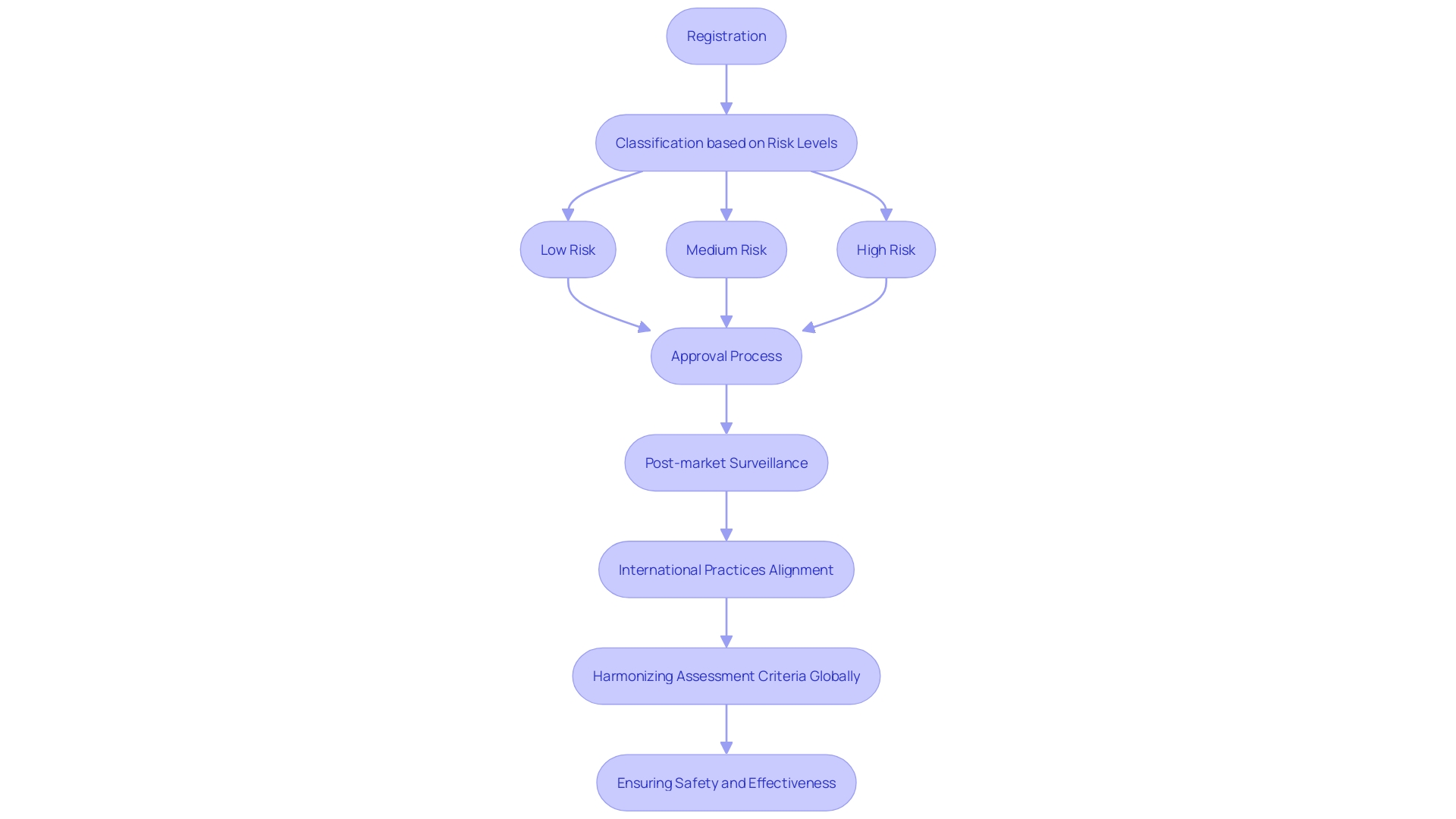
Classification of Medical Devices in Argentina
In Argentina, medical instruments are categorized into four classes based on their risk levels: Class I, Class II, Class III, and Class IV. Class I items, posing the least risk, require minimal regulatory oversight. In contrast, Class IV products, associated with the highest risk, undergo a stringent review process. This classification framework is pivotal for determining the necessary clinical trials, quality management systems, and post-market surveillance. Understanding these regulatory pathways is essential for manufacturers and researchers aiming to successfully enter and operate in the Argentine market. 'This process, akin to the FDA's establishment registration in the United States, involves identifying the correct classification of the product to ensure compliance and streamline market entry.'. Furthermore, remaining aware of changing regulatory requirements and technological progress in healthcare product development can greatly improve the success and effectiveness of maneuvering through these intricate environments.
Clinical Trial Requirements for Medical Devices
Carrying out medical studies for healthcare equipment in Argentina requires strict compliance with rules set by the National Administration of Drugs, Foods and Medical Devices (ANMAT). A thorough research protocol must be carefully created and submitted for authorization before any study can begin. Moreover, it is essential that these examinations conform to Good Clinical Practice (GCP) standards and maintain ethical considerations to prioritize participant safety and rights. This process mandates obtaining informed consent from all participants and securing approval from an ethics committee, which rigorously evaluates the ethical implications of the proposed study. By integrating these measures, Argentina ensures that medical studies are conducted with the utmost integrity and respect for all involved.
Good Clinical Practice (GCP) and Ethical Considerations
Good Clinical Practice (GCP) is a fundamental aspect of conducting studies for medical devices in Argentina. GCP includes guidelines that guarantee the integrity of data gathered during a study and the safety of participants. Ethical considerations are paramount, requiring researchers to uphold principles such as respect for persons, beneficence, and justice. These principles direct the creation and execution of research studies, highlighting the significance of openness, participant enrollment, and informed consent procedures.
In recent years, decentralized clinical studies (DCTs) have emerged as a significant innovation in clinical research. Utilizing digital technologies, DCTs tackle the limitations of conventional site-based studies, which frequently encounter obstacles such as geographical restrictions, insufficient diversity among participants, and logistical difficulties.
'Conventional studies usually necessitate that participants journey to designated places for screening, intervention, and follow-up procedures, which can exclude individuals residing in isolated regions or those lacking sufficient transportation options.'. This approach limits the diversity and representativeness of the study population, particularly affecting historically marginalized groups.
In contrast, DCTs provide a more inclusive method by enabling participants to take part from their homes or local healthcare facilities. This method improves participant involvement and retention, ensuring a broader and more diverse study population. The application of digital instruments in DCTs not only enhances better access but also simplifies data gathering, boosting the overall efficiency and effectiveness of medical studies.
Furthermore, the incorporation of artificial intelligence and real-world data in research studies can strengthen regulatory submissions, as discussed at the Outsourcing in Clinical Studies: Medical Devices Europe 2024 conference. These advancements emphasize the continuous initiatives to enhance medical studies, rendering them more available and reflective of various populations.
Ensuring the ethical conduct of these studies remains crucial. Researchers must present informed consent in a way that facilitates understanding, emphasizing the importance of transparency regarding the purpose, risks, benefits, and procedures of the study. Respect for participants' privacy and minimizing the impact on their physical and mental integrity are essential aspects of upholding ethical standards in research.
Challenges and Opportunities in Argentina's Clinical Trial Landscape
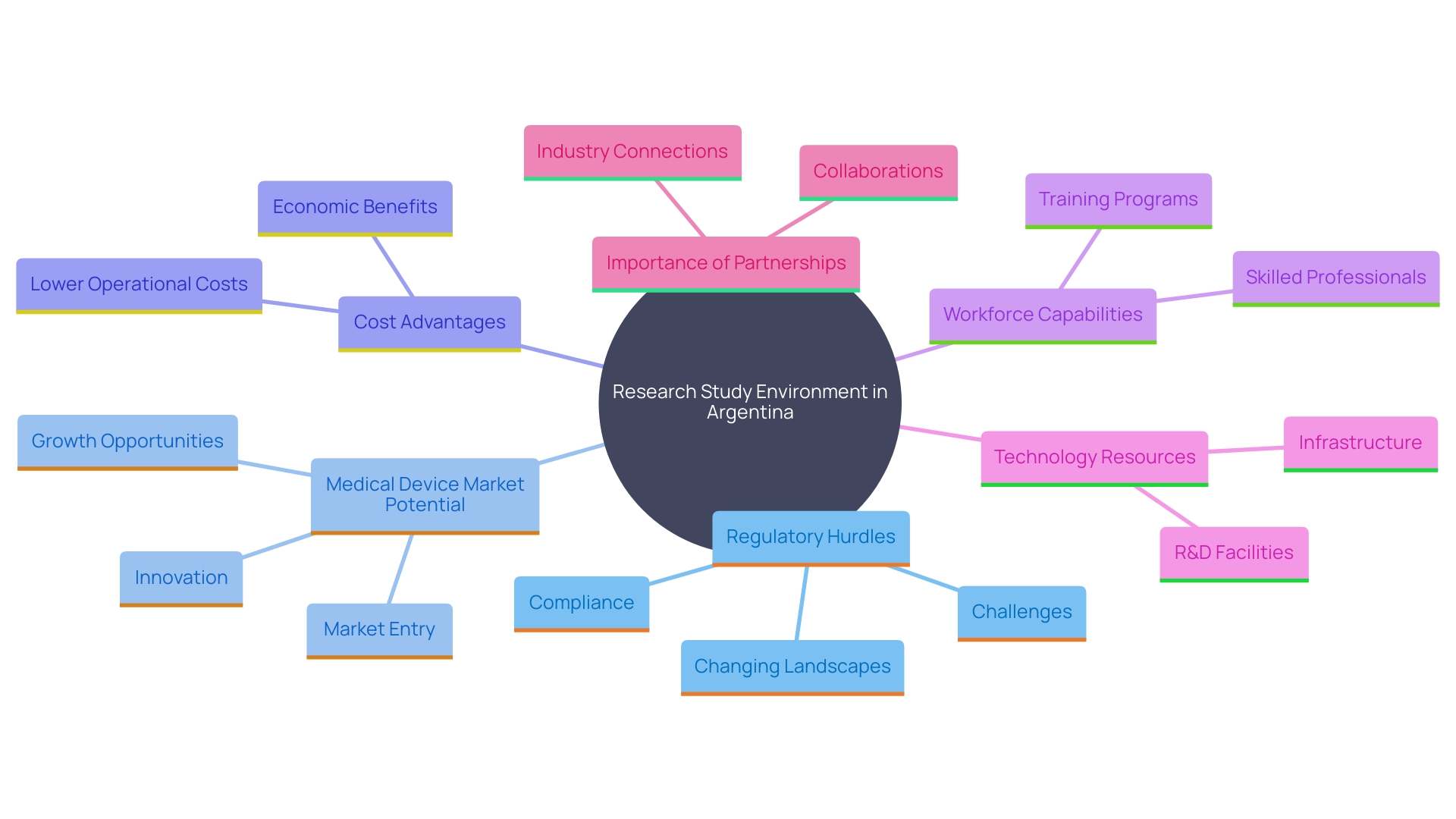
The research study environment in Argentina highlights a mix of difficulties and possibilities. Despite regulatory hurdles and bureaucratic processes that might hinder the swift initiation of studies, Argentina's expanding medical device market offers substantial potential for innovation and collaboration. The relatively low infrastructure, procedural, and labor costs, compared to developed countries, make Argentina an attractive destination for international industries. Moreover, the existence of a proficient workforce and rising investments in healthcare establish a favorable atmosphere for carrying out research in a medical setting.
A notable benefit is the chance for technology resources to alleviate typical research challenges. These supplies can address issues like protocol design errors, slow recruitment, personnel training, and monitoring, as well as data management, analysis, and reporting. This is particularly significant for phase I studies, where speeding up the timeframe from study implementation to database lock is crucial.
Additionally, partnerships between local firms and international companies can facilitate knowledge transfer and enhance the overall research ecosystem. This cooperative atmosphere is crucial for progressing health technology and making certain that research studies are carried out efficiently and effectively. By utilizing these collaborations and tackling the current obstacles, Argentina can bolster its role as a significant participant in the worldwide research landscape.
Growth and Trends in Argentina's Medical Device Clinical Trials Market
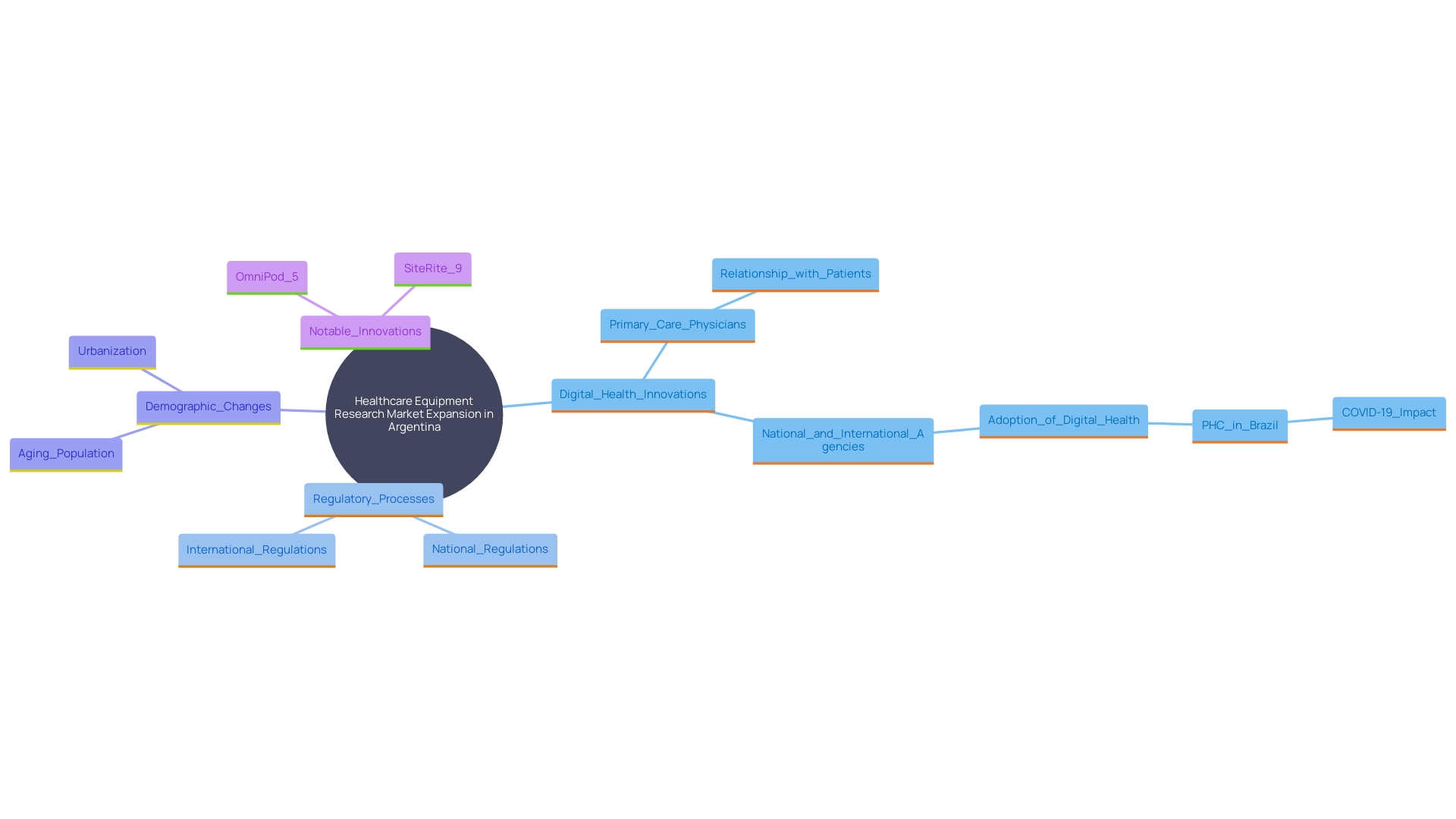
The healthcare equipment research market in Argentina has seen significant expansion, driven by the rising need for innovative health solutions. A key reason for this increase is the growing emphasis on digital health innovations and minimally invasive tools, which correspond with worldwide trends in healthcare technology. Furthermore, as regulatory processes in Argentina become more efficient, the nation is anticipated to attract more international sponsors keen to perform trials. This trend emphasizes the essential requirement for thorough research practices in healthcare to guarantee the successful creation and marketing of health-related devices.
One prominent example of this growth is the expanding market for tissue-engineered skin substitutes in Argentina. Changes in population demographics, disease prevalence, and macroeconomic factors all influence market dynamics. Moreover, shifts in healthcare practice aimed at improving medical outcomes can lead to changes in diagnosis and treatment, further impacting the market. For instance, Insulet’s OmniPod 5 Automated Insulin Delivery System and the SiteRite 9 Ultrasound System are notable innovations contributing to the evolving landscape.
Moreover, establishing medical studies in smaller nations such as Argentina provides various benefits, such as increased recruitment numbers and access to a more involved research personnel. According to Carine Cochereau, vice president of regulatory international at Integra Life Sciences, these benefits were discussed at the Outsourcing in Clinical Trials: Medical Devices Europe 2024 conference. The conference also highlighted the potential of artificial intelligence and real-world data to enhance regulatory submissions, underscoring the importance of optimizing clinical trials to maintain Argentina's growing appeal as a clinical research hub.
Conclusion
The regulatory framework for medical devices in Argentina, spearheaded by ANMAT, provides a thorough and systematic approach to ensuring safety and efficacy. This framework is crucial for the registration, classification, and post-market surveillance of medical devices, establishing a robust compliance system that aligns with international standards. With a classification system that categorizes devices based on risk levels, manufacturers can better navigate the regulatory landscape, facilitating smoother market entry.
Clinical trials in Argentina are governed by stringent guidelines that emphasize Good Clinical Practice (GCP) and ethical considerations. The necessity for a comprehensive clinical trial protocol, informed consent, and ethics committee approval underscores the commitment to participant safety and data integrity. The emergence of decentralized clinical trials represents a significant innovation, enhancing accessibility and diversity in participant recruitment, thereby addressing traditional trial limitations.
Despite the challenges posed by regulatory hurdles, Argentina's medical device market presents numerous opportunities for growth and collaboration. The country’s relatively low operational costs, combined with a skilled workforce and increasing healthcare investments, create a favorable environment for clinical research. Partnerships between local and international firms can further enhance the research ecosystem, driving innovation and efficiency.
As the medical device clinical trials market continues to expand, driven by digital health technologies and innovative solutions, Argentina is poised to strengthen its position as a key player in the global landscape. The anticipated improvements in regulatory processes will likely attract more international sponsors, emphasizing the need for rigorous clinical research practices to ensure the successful development and commercialization of medical devices.




