Introduction
In clinical trials, the identification and classification of adverse events are crucial for ensuring participant safety and the integrity of the trial. From mild reactions to severe conditions, adverse events can vary widely in their impact. This article explores the definition and classification of adverse events, including the distinction between serious and non-serious events.
It also delves into the criteria for reporting serious adverse events and the role of clinical research associates in monitoring these events. Additionally, the article discusses the documentation and reporting requirements for adverse events and the overlap between adverse events and serious adverse events in clinical trials. The impact of adverse events on clinical trial outcomes is examined, highlighting the need for meticulous analysis and interpretation of data.
Furthermore, the article explores the use of predictive models, leveraging artificial intelligence, to identify serious clinical outcomes resulting from adverse reactions. Through a comprehensive understanding of adverse events and their implications, researchers can navigate the complex landscape of clinical trials while prioritizing participant safety and advancing medical knowledge.
Definition and Classification of Adverse Events
In clinical trials, the term 'adverse event' refers to any undesirable experience associated with the use of a medical product in a patient. The spectrum of these events can vary widely, from mild, transient reactions to severe, life-altering conditions. Proper identification and categorization of these events are vital for the integrity of the trial and for ensuring the safety of participants. For example, during Pfizer's COVID-19 vaccine trials, a participant named Maddie experienced severe sickness, which raised concerns about the vaccine's safety and the transparency of its reporting. Such cases underscore the importance of rigorous adverse event monitoring systems like the Vaccine Adverse Event Reporting System (VAERS), which has been instrumental in identifying vaccine-related issues. Trials for the COVID-19 mRNA vaccines, which showed a remarkable 95% relative risk reduction in symptomatic COVID-19, were conducted under unprecedented timelines, with Pfizer's vaccine reaching market authorization in just seven months, compared to the previous average of 10 years for phase 3 trial testing. This accelerated process, while successful in addressing an urgent global health crisis, also emphasizes the need for ongoing post-marketing surveillance to ensure long-term vaccine safety.
Types of Adverse Events: Serious vs. Non-Serious
In clinical trials, adverse events are meticulously recorded to ensure the safety of participants and the efficacy of the intervention under study. These events are broadly classified into serious adverse events (SAEs) and non-serious adverse events. SAEs are defined by their grave outcomes, which may include death, a threat to the patient's life, a necessity for hospitalization, enduring disability, or other significant health disruptions. For instance, the story of a 70-year-old man who quickly deteriorated from being an avid walker to nearly incapacitated underscores the seriousness of such events, as his condition could have qualified as a SAE had it occurred during a clinical trial.
On the other hand, non-serious adverse events, while still important, do not pose an immediate threat to a patient's life or long-term health. These can often be managed without the need for significant medical intervention. However, the distinction between serious and non-serious can sometimes be blurred, as was seen in the controversial case of Maddie, a participant in the Pfizer COVID-19 vaccine study who experienced severe illness post-vaccination. Her case highlights the intricate challenges of monitoring and classifying adverse events in real-world scenarios.
Clinical trials are the cornerstone for assessing the safety and effectiveness of new medical interventions. Recent trials of mRNA COVID-19 vaccines by Pfizer-BioNTech and Moderna have demonstrated a remarkable 95% relative risk reduction in symptomatic COVID-19, leading to an unprecedented Emergency Use Authorization by the FDA. This swift progress, compared to the traditional 10-15 year timeline for vaccine safety evaluation, exemplifies the dynamic nature of clinical trial safety assessment and the importance of vigilance in monitoring adverse events.
For researchers, the primary goal is to ensure the safety of participants while seeking to answer vital research questions. As SAEs can have far-reaching implications on the outcome and credibility of a trial, they are closely scrutinized and documented, serving as a critical component of the broader national vaccine safety system, as emphasized by the success of VAERS in identifying vaccine-related safety issues.
Criteria for Reporting Serious Adverse Events
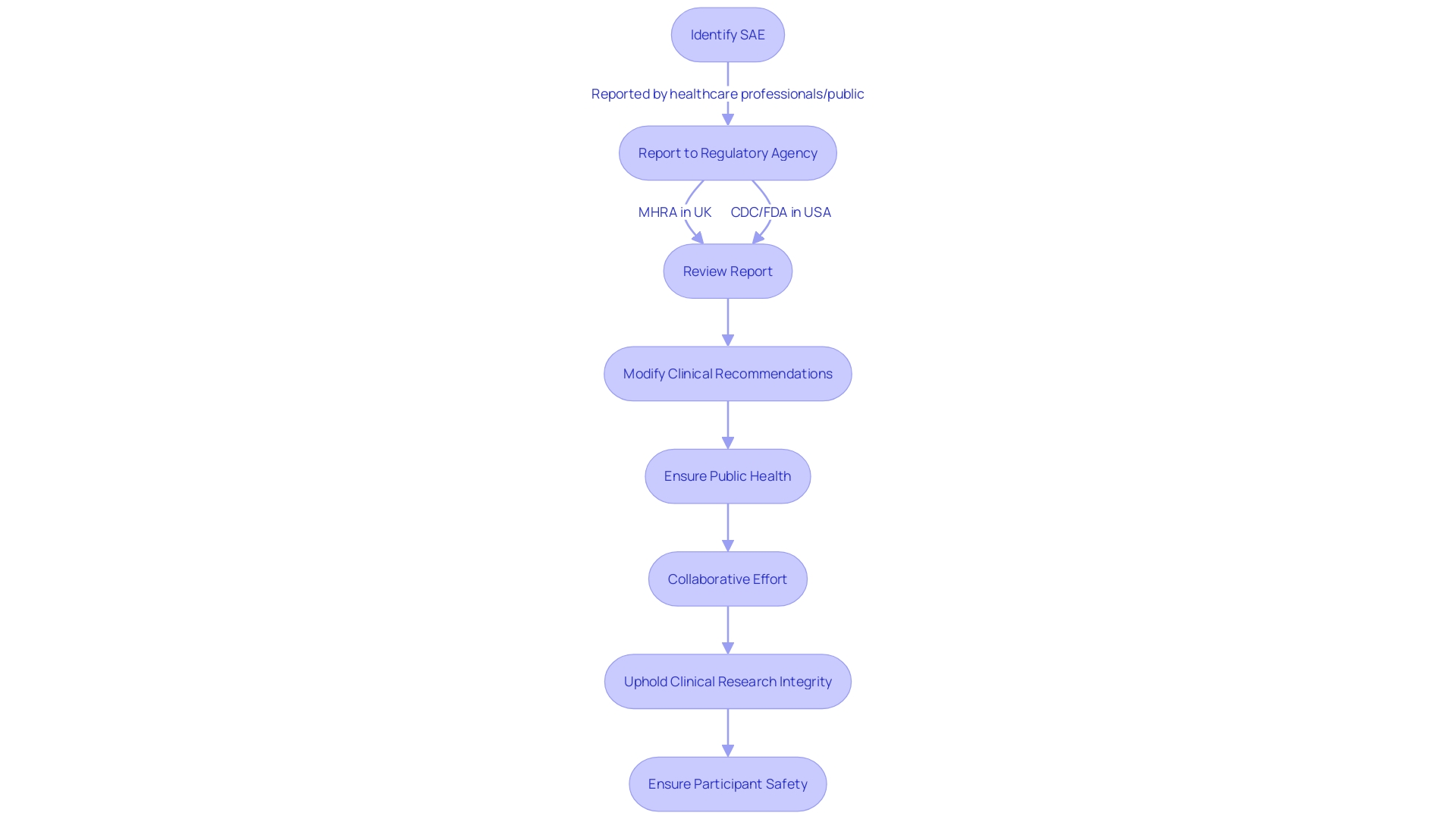
In the realm of clinical trials, the prompt and accurate reporting of serious adverse events (SAEs) is paramount for participant safety. This process is guided by stringent regulatory criteria, which are tailored to the specific nature of each trial. For instance, the Yellow Card Scheme (YCS), instituted in 1964, serves as a sentinel system in the United Kingdom for the detection of unforeseen adverse drug reactions (ADRs). Through YCS, health professionals and the public can submit reports of suspected ADRs to the Medicines and Healthcare products Regulatory Agency (MHRA), contributing to the safeguarding of medicine and medical device utilization. A survey of YCS participants revealed that a substantial number of individuals—nearly half—were informed of the reporting system through pharmacies, illuminating the crucial role of community healthcare providers in post-market surveillance.
Similarly, in the United States, the Vaccine Adverse Event Reporting System (VAERS) operates as a crucial component of the national vaccine safety monitoring network. VAERS enables the collection and analysis of information regarding adverse events that occur after vaccination. As a testament to its efficacy, VAERS has been instrumental in identifying safety concerns, thereby reinforcing the safety profile of vaccines. It exemplifies a collaborative effort between the Centers for Disease Control and Prevention (CDC) and the Food and Drug Administration (FDA), where experts scrutinize reports and, if necessary, modify clinical recommendations to ensure public health.
Moreover, the vigilance in reporting SAEs is not confined to healthcare professionals alone. Public engagement plays a vital role, with 81% of respondents in a YCS survey indicating that the decision to report was initiated by themselves. This active participation underscores the value of public-sourced data in enhancing drug safety.
These reporting systems, albeit not indicative of causality on their own, stand as critical early-warning mechanisms. They facilitate the identification of potential risks, allowing for the investigation and, when warranted, prompt regulatory action to mitigate any harm to patients. It's this level of proactive surveillance and community involvement that upholds the integrity of clinical research and the safety of its participants.
Role of Clinical Research Associates in Monitoring Adverse Events
Clinical research associates (Cras) play a pivotal role in the safety monitoring of participants during clinical trials. Their responsibilities extend beyond mere data collection; they are tasked with the critical function of ensuring the precise and prompt documentation of adverse events. Moreover, Cras engage in an evaluative process to determine the severity of these events and their potential implications for the trial's progression. This rigorous oversight is vital in preserving the integrity of collected data and safeguarding participant welfare.
These professionals liaise closely with investigators and study coordinators, forming a collaborative effort to uphold the highest standards of data quality. For instance, the research team at the NIH's Neuro-Oncology Clinic exemplifies the integration of patient education and clinical trial management, ensuring that participants are fully informed and prepared for the implications of trial involvement. This comprehensive approach is crucial in managing the dual aspects of excitement and uncertainty inherent in clinical trials, particularly in studies involving rare brain and spine tumors.
Additionally, the use of platforms like The New Normal for participant recruitment demonstrates the innovative strategies that Crash and research teams employ to attract and retain trial participants. These platforms not only facilitate increased awareness and access to health research opportunities but also underscore the importance of participant education and engagement in the research process.
In the dynamic field of clinical research, Crash and their collaborative networks play a fundamental role in advancing medical knowledge while prioritizing participant safety and well-being. The experiences of individuals like Barbara, who discovered critical health information through trial participation, highlight the dual benefit of clinical trials in contributing to personal health insights and broader medical advancements.
Documentation and Reporting Requirements for Adverse Events
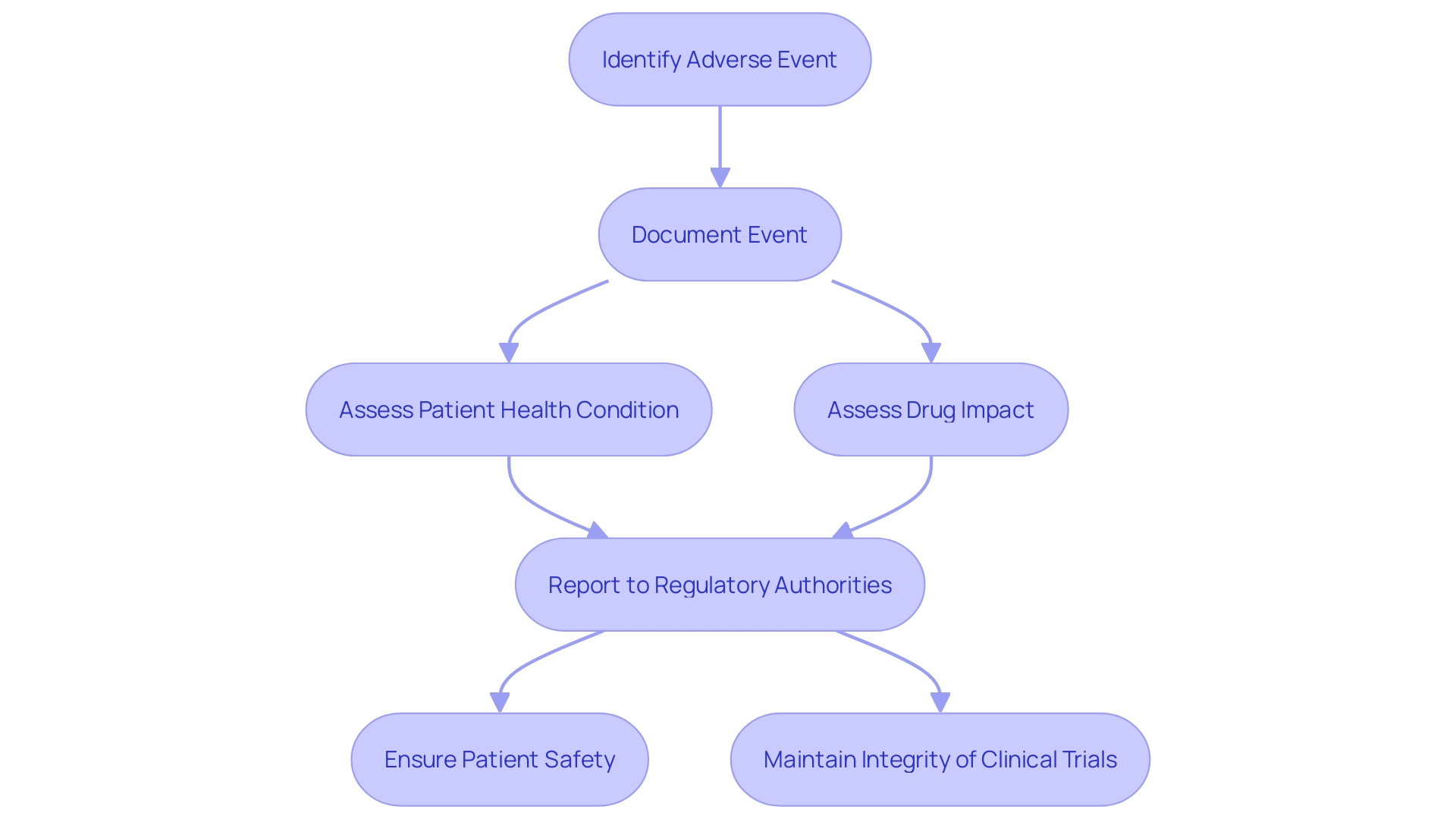
The recording and reporting of adverse events in clinical trials is a critical task that ensures the safety and efficacy of new medicinal products. Clinical researchers, such as those led by Osipenko and Gamertsfelder, play a pivotal role in this process. They meticulously document each adverse event, considering the patients' underlying health conditions and the potential impact of the investigational drug. These observations are particularly vital in trials involving vulnerable populations, such as the elderly with multiple health conditions or those with transthyretin-mediated amyloidosis, as described in recent case studies.
The significance of these efforts is underscored by FDA regulations, which mandate clear and conspicuous communication of drug side effects and contraindications in direct-to-consumer advertisements. Similarly, the Vaccine Adverse Event Reporting System (VAERS) demonstrates the importance of monitoring safety, having been instrumental in identifying vaccine safety concerns. The system's success is a testament to the value of adverse event reporting, reinforcing the need for vigilance in clinical trials.
Clinical trial documentation encompasses various aspects, from consent requirements for different populations to the specifics of investigational products. Moreover, standard reporting structures, such as the Development Safety Update Report (DSUR), provide a systematic approach to summarizing safety data. This includes details about serious adverse events (SAEs) and any new safety signals that may emerge, ensuring that researchers and regulators can make informed decisions about the continuation and approval of clinical trials.
In this context, the role of investigators and study coordinators extends beyond mere data collection; they are guardians of patient safety and integrity in the pursuit of medical advancements.
Case Study: Overlap between Adverse Events and Serious Adverse Events in Clinical Trials
A poignant example of the complex nature of adverse events in clinical trials can be drawn from the experience of a family participating in Pfizer's vaccine study for children. Stephanie de Garay's children were enrolled in the trial at Cincinnati Children's Hospital when her daughter Maddie became severely ill. This incident underscores the critical need for vigilant monitoring and reassessment of adverse events, as mild symptoms may escalate, becoming serious adverse events requiring hospitalization. Such events can also raise questions about the integrity of the trial, as seen in the allegations that Pfizer and regulatory bodies tried to dismiss Maddie's case. Moreover, the significant failure rate of clinical trials, which stands at around 90%, not only inflates research and development costs to about $1 billion for a new drug but also emphasizes the importance of rigorous trial design and sample size estimation. These challenges highlight the delicate balance required to advance patient care while ensuring the safety and efficacy of new treatments. The narrative around Maddie's case reflects broader concerns in clinical research, which include potential underpowered studies due to optimistic estimations of event rates and effect sizes. This further illustrates the necessity of maintaining the highest standards of data integrity and ethical conduct in clinical trials.
Impact of Adverse Events on Clinical Trial Outcomes
Serious Adverse Events (SAEs) are critical concerns in clinical trials, as they can drastically affect the course and outcomes of studies. The occurrence of SAEs might necessitate alterations in the treatment protocol or dosage adjustments, potentially impacting the trial results. It is essential for researchers to meticulously analyze and interpret data in light of SAEs' potential influences.
The gravity of SAEs is highlighted by the case of a major contract research organization (CRO), Fortrea, which faced allegations of errors in a clinical trial they managed. Despite Fortrea's long-standing reputation for integrity and over 30 years of experience in conducting clinical trials, this incident underscores the importance of diligent oversight and quality control in all aspects of trial management, particularly when third-party vendors are involved.
Randomization is a fundamental aspect of clinical trials, ensuring unbiased distribution of treatments across study participants. However, the integrity of this process can be compromised by errors, such as the 'programming error' reported in a hidradenitis suppurativa trial. Such mistakes can lead to the misadministration of doses and invalidate trial designs, directly affecting patient outcomes and the credibility of study results.
Understanding the ethical framework that governs clinical research is crucial. The Declaration of Helsinki and The Belmont Report establish principles of respect, beneficence, and justice that remain at the forefront of ethical considerations. Moreover, the evolving landscape of clinical trials has prompted the Multi-Regional Clinical Trials Center of Brigham and Women's Hospital and Harvard to release updated guidance on the return of individual data and results to participants.
The challenge of balancing participant safety with trial integrity is further complicated by public health emergencies. The FDA has expanded its guidance to encompass a broad range of emergencies, focusing on risk-based evaluations for monitoring clinical trial sites and subjects. This shift reflects the need to adapt protocols in the face of disruptions, such as those experienced during the COVID-19 pandemic, which had lasting impacts on trial execution and the evaluation of safety and efficacy.
Furthermore, the complexity of clinical trials is on the rise, with the average Phase 3 trial now involving numerous visits and procedures for each participant. This complexity, alongside the expectation of many participants to receive their personal results, adds to the challenges faced by researchers in providing trial data to patients.
Statistics reveal the increasing duration and complexity of clinical trials, with up to 80% of studies failing to finish on time, and the length of Phase 3 trials extending from 41 to 44 months. The urgency of clinical development, coupled with competitive pressures and regulatory changes, such as the US Inflation Reduction Act, highlights the importance of timely and efficient trial management.
In conclusion, SAEs are a pivotal aspect of clinical trials that researchers must navigate with precision and ethical consideration. The potential repercussions of Sales on trial outcomes and the broader implications of ethical conduct, regulatory compliance, and participant engagement in the research process are fundamental considerations for the successful execution of clinical trials.
Predictive Models for Identifying Serious Clinical Outcomes of Adverse Reactions
The pursuit of enhanced patient care and outcomes in clinical trials has led to the integration of artificial intelligence (AI) into the prediction and management of adverse events. Predictive models leveraging AI are progressively being refined to forecast serious clinical outcomes resulting from adverse reactions with greater precision. These models incorporate a multitude of variables, such as patient demographics, medical history, and specific characteristics of the adverse event, to calculate the probability of a serious clinical outcome.
Recent studies have underscored the potential and the challenges associated with these AI-driven models. For instance, machine learning models have demonstrated impeccable performance within the confines of the datasets they were trained on, which often include extensive international multisite clinical trials. Despite this, their efficacy significantly diminishes when applied to independent patient samples in new clinical settings, often plummeting to levels comparable to random chance. This discrepancy reveals a critical gap in the prospective testing of such models and highlights the need for further validation before they can be reliably used.
Beyond predictive performance, the application of large language models (LLMs) has shown promise in enhancing the precision and efficiency of identifying complications such as immune-related adverse events (irAEs) in hospital settings. Unlike traditional methods, which are manual and labor-intensive, LLMs can sift through extensive datasets to detect irAEs with higher accuracy than retrospective methods like International Classification of Disease (ICD) codes.
These advancements come amid a wider context where the Food and Drug Administration (FDA) employs diverse data sources, including clinical trials, to inform postmarketing safety surveillance. The Sentinel System, as part of the FDA's commitment, utilizes curated healthcare data to generate timely evidence regarding medication safety.
Nonetheless, the success of these AI applications is not without its challenges. The high failure rate of clinical trials contributes significantly to the substantial research and development costs of new drugs, with a large proportion of trials failing to reject the null hypothesis or presenting inconclusive results due to potential type II errors. These setbacks are often attributed to underpowered studies caused by optimistic estimations of event rates and effect sizes, highlighting the importance of robust and accurate predictive tools.
As the clinical research landscape continues to evolve, the integration of AI into the management of adverse events in clinical trials represents a promising avenue. It aims not only to improve the prediction of serious clinical outcomes but also to streamline the identification of complications, thereby potentially enhancing patient care and the efficiency of trial conduct.

Conclusion
In conclusion, the identification and classification of adverse events are crucial in clinical trials to ensure participant safety and trial integrity. Adverse events can range from mild reactions to severe conditions, highlighting the need for meticulous monitoring and documentation. The distinction between serious and non-serious adverse events is important, as serious events can have significant impacts on patient health and trial outcomes.
The prompt reporting of serious adverse events is paramount, guided by stringent regulatory criteria and systems such as the Yellow Card Scheme and the Vaccine Adverse Event Reporting System. Clinical research associates play a vital role in monitoring adverse events, ensuring precise documentation and evaluation of their severity. The documentation and reporting of adverse events are critical for assessing the safety and efficacy of new medicinal products, with FDA regulations and reporting structures like the Development Safety Update Report providing systematic approaches.
The overlap between adverse events and serious adverse events can present challenges in monitoring and classifying events accurately. Serious adverse events can significantly impact trial outcomes, necessitating careful analysis and interpretation of data. Predictive models leveraging artificial intelligence show promise in identifying serious clinical outcomes resulting from adverse reactions, although further validation is needed.
The integration of AI into adverse event management aims to enhance patient care and trial efficiency. Overall, the comprehensive understanding of adverse events and their implications is essential for prioritizing participant safety and advancing medical knowledge in clinical trials.




