Introduction
The Corrective and Preventive Actions (CAPA) system plays a critical role in the field of medical device manufacturing, ensuring product integrity and patient safety. By proactively identifying and addressing issues, CAPA promotes continuous improvement and adherence to regulatory requirements. This article explores the key components of a well-designed CAPA system, provides a step-by-step guide to the CAPA process, discusses FDA requirements for CAPA systems, highlights essential elements of an effective CAPA system, identifies common CAPA problems to avoid, and emphasizes the role of CAPA in quality management systems.
Additionally, the article presents best practices for implementing and maintaining CAPA systems in medical device manufacturing. By following these best practices, manufacturers can enhance compliance, improve product quality, and prioritize patient safety.
What is CAPA?
Corrective and Preventive Actions (CAPA), an acronym that stands for measures taken to identify, analyze, and rectify issues affecting product integrity and patient safety, plays an essential role in the field of manufacturing devices for healthcare. This methodology is not just a reactive measure—it also proactively anticipates potential problems, ensuring continuous improvement in product quality and process efficiency.
At the heart of the process is a sequence of actions that involve identifying the problem, conducting a thorough analysis of the underlying causes, creating corrective measures, implementing them, and continuously monitoring their effectiveness. This comprehensive process is integral to maintaining rigorous standards in device production and adhering to regulatory requirements.
An actual instance of corrective and preventive actions in operation can be observed in the scenario of an Australian technological startup that recognized a void in the industry for an inexpensive manufacturing implementation solution customized to the healthcare sector. In response to government mandates for meticulous tracking and auditing of production processes, the company collaborated with Facile Technolab to develop a SaaS platform. This system enables small to medium-sized enterprises to manage compliance efficiently, illustrating how CAPA principles can extend to broader operational challenges and foster innovation.
Industry trends suggest a rise in the automation and digitalization of healthcare, especially in the assembly of healthcare equipment. As the regulatory landscape evolves, manufacturers must adapt by employing appropriate tools and forming strategic partnerships to navigate this complex terrain. Embracing automation and AI is predicted to be critical for the sector's future, with meticulous compliance being a non-negotiable aspect of successful implementation.
The accuracy needed in manufacturing healthcare equipment is unparalleled, requiring strict compliance with standards from the beginning of the design process to the final quality assessment. The role of equipment in this process is pivotal, especially in executing exacting measurements and inspections. These metrological practices are crucial to the effectiveness of medical instruments, emphasizing the importance of corrective and preventive actions in guaranteeing devices comply with precise requirements and function efficiently within the human body.
Considering the importance of design changes, a professional in the field remarked, 'Any alteration to your equipment will always necessitate documentation and may even call for a fresh regulatory submission.' This emphasizes the significance of carefully documenting every modification, whether it comes from customer input, changes in materials, or adjustments in manufacturing, supporting the vitality of managing such changes.
To sum up, Corrective and Preventive Actions (CAPA) are not just a regulatory necessity; they represent a strategic methodology that guarantees the dependability and security of healthcare instruments, thus protecting the health and welfare of patients.
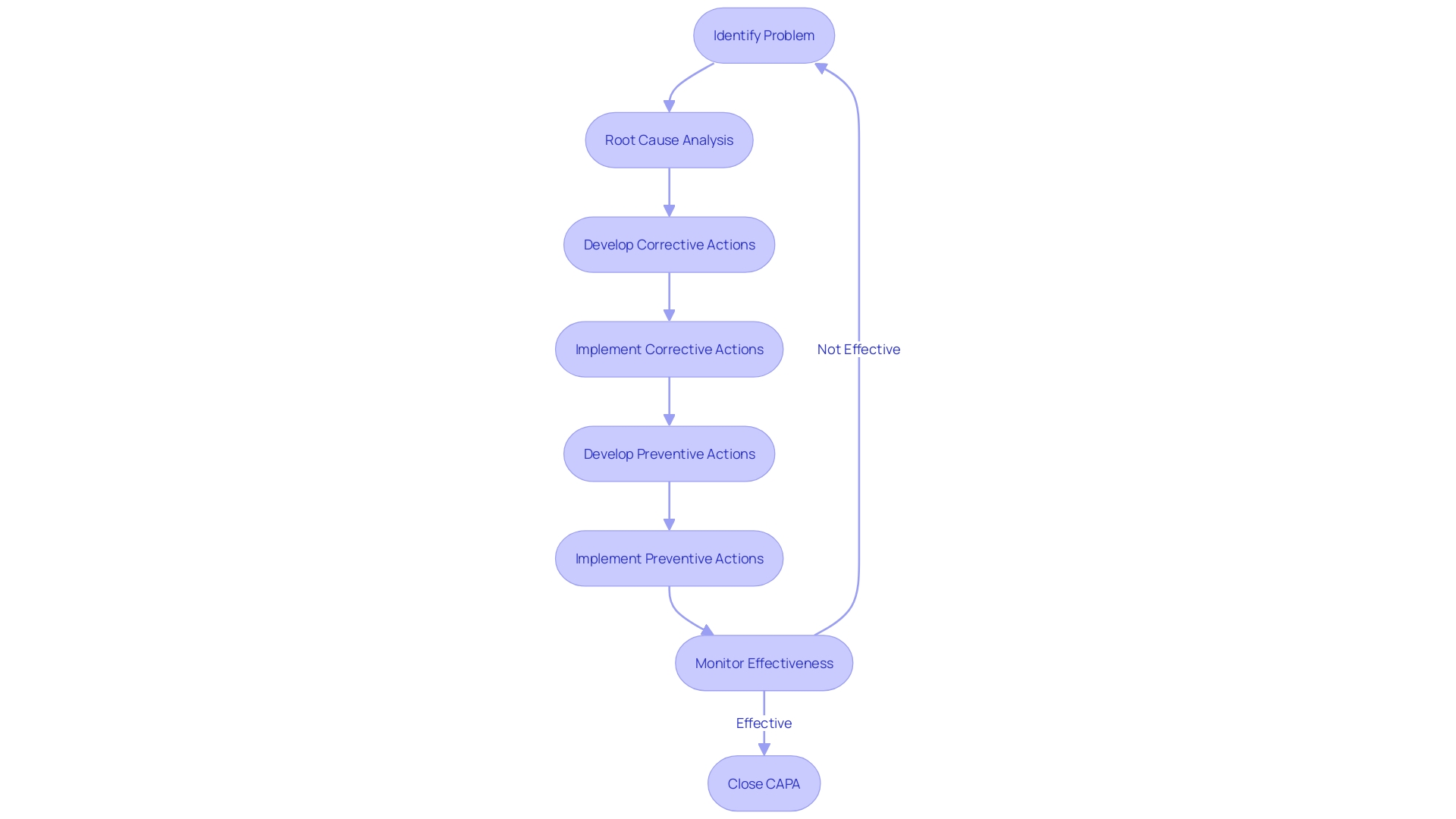
Key Components of a CAPA System
In the ever-evolving realm of medical device manufacturing, a strong Corrective and Preventive Action (CAPA) mechanism is essential for upholding superior levels of quality and safety. A corrective and preventive action system is designed to effectively address issues, prevent recurrence, and ensure continuous improvement. Here are the essential components of a well-designed CAPA system:
-
Problem Identification: This step involves a meticulous process of recognizing and documenting any deviations or irregularities in the medical device or its production process. It's imperative that the identification is systematic to capture all potential issues.
-
Root Cause Analysis: Following the identification of an issue, an in-depth root cause analysis is performed. This critical phase uncovers the fundamental reasons behind the problem, paving the way for focused corrective and preventive measures.
-
Corrective and Preventive Actions: With the insights gained from the root cause analysis, suitable corrective and preventive actions are formulated. Corrective actions are tailored to resolve the current issue, and preventive actions are conceptualized to avert similar future occurrences.
-
Implementation and Verification: The devised actions are then put into effect, and their efficacy is assessed through vigilant monitoring and rigorous data evaluation. This verification is essential to confirm the resolution of the identified issues.
-
Complete documentation and monitoring of the corrective and preventive action process is crucial. This includes recording all stages from problem identification, through root cause analysis, to the development and verification of actions. Such records are indispensable for ongoing monitoring, regulatory audits, and evidence of due diligence in maintaining quality and safety standards.
An example illustrating the importance of flexible and expandable corrective and preventive action mechanisms is the project undertaken by Facile Technolab. They worked together with a tech startup from Australia to create a minimum viable product for the manufacturing industry in healthcare, focusing on a crucial requirement for a cost-effective manufacturing execution solution that meets government regulations for monitoring and reviewing. This project not only met the specific requirements of a client but also established a model that could be tailored and extended for more clients, demonstrating a progressive approach to design of corrective and preventive action systems.
Moreover, recent progress in testing of healthcare equipment, like the ones conducted at UL Solutions' laboratory in Michigan, highlights the industry's dedication to safety and excellence. The facility is equipped to handle a variety of testing methods, adhering to manufacturers' specifications and maintaining compliance with pertinent performance and safety standards.
In summary, a corrective and preventive action framework is essential for manufacturers of healthcare equipment to navigate the intricate environment of equipment variety and regulatory adherence. The process must be comprehensive, from identifying issues to implementing and documenting corrective measures, to maintain the integrity and dependability of healthcare equipment in the market.
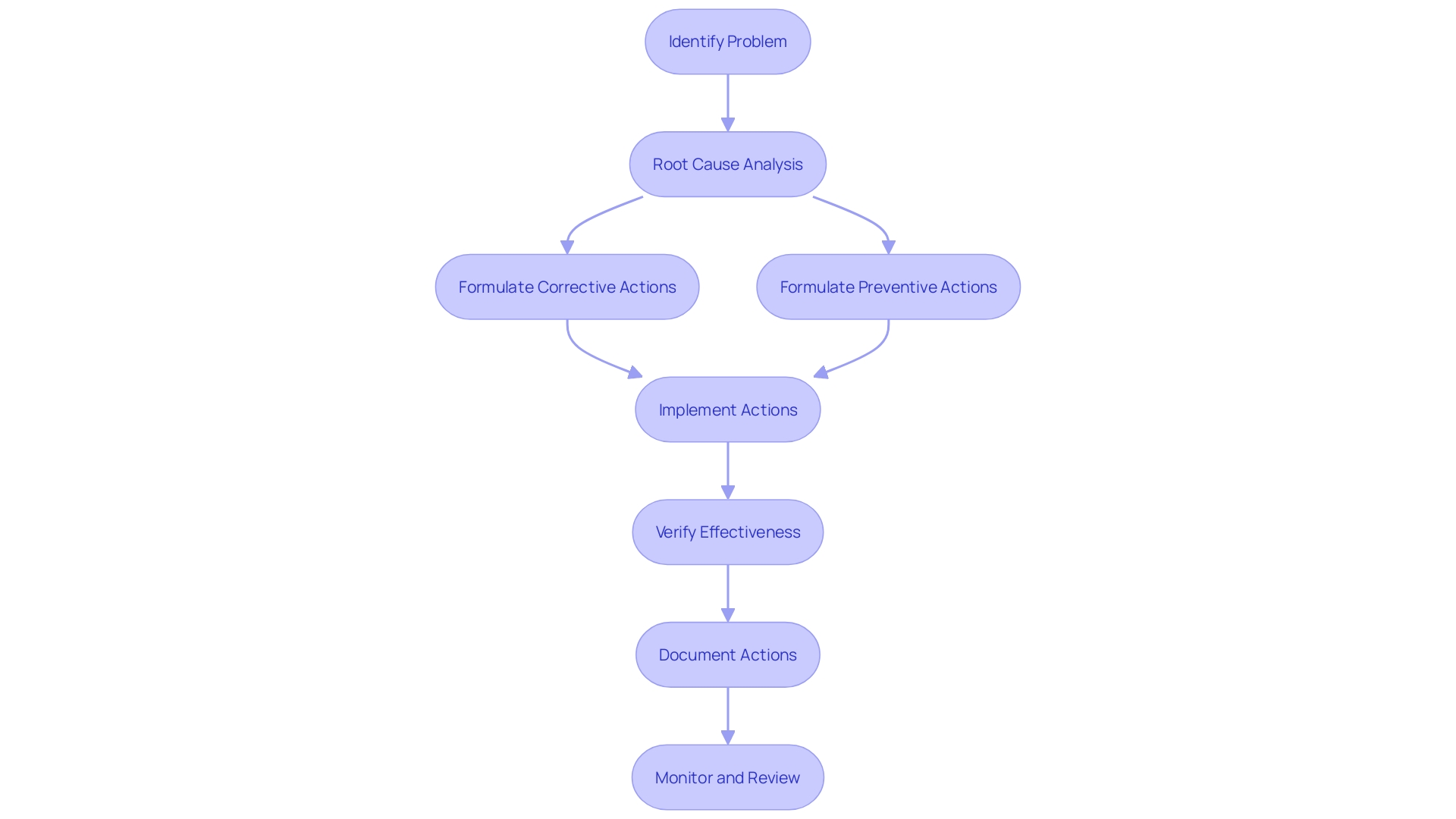
CAPA Process: Step-by-Step Guide
The adoption of a Corrective and Preventive Action (CAPA) mechanism is a systematic approach to tackle potential and actual matters in healthcare equipment systems and procedures. Here's an elucidation of the procedure:
-
Identification and Description of the Problem: The initial phase demands recognizing and characterizing the issue, which could stem from consumer grievances, internal audits, or alternative sources. For example, in the NHS, clinicians submit a request form for new digital technology to the Digital Service Team for an initial assessment, ensuring that it is secure, appropriate, and meets compliance standards.
-
Root Cause Analysis: After pinpointing the problem, a meticulous root cause analysis is essential. This involves rigorous data collection, investigations, and the use of problem-solving tools. The process mirrors the approach taken by the Digital Service Team, scrutinizing whether the technology in question is redundant or already in use elsewhere.
-
Development of Corrective and Preventive Actions: Leveraging the insights from the root cause analysis, we then formulate corrective and preventive measures that are SMART - specific, measurable, achievable, relevant, and time-bound. These actions are akin to the careful consideration of new technologies in healthcare, ensuring they address current needs without duplicating existing solutions.
-
Implementation and Verification of Actions: The devised strategies are executed, followed by a rigorous verification process through monitoring and data assessment. This stage can involve experimental trials, similar to UL Solutions' Rochester Hills laboratory, which customizes testing to comply with manufacturers' methods and specifications.
-
Documentation and Tracking of CAPA Activities: Documenting and tracking the CAPA endeavors is imperative throughout the entire process. This organized record-keeping is reminiscent of the way ALVAMED, a leader in equipment consulting, adapts its support levels to the evolving needs of clients, ensuring compliance and quality assurance at every stage.
In the healthcare technology industry, these steps emphasize the significance of digitalization and the ongoing development of healthcare technology, as emphasized by the convergence of digital and physical in healthcare design. It's a realm where the form, fit, and function of equipment are constantly evaluated and improved upon to meet stringent regulatory standards and enhance patient care.
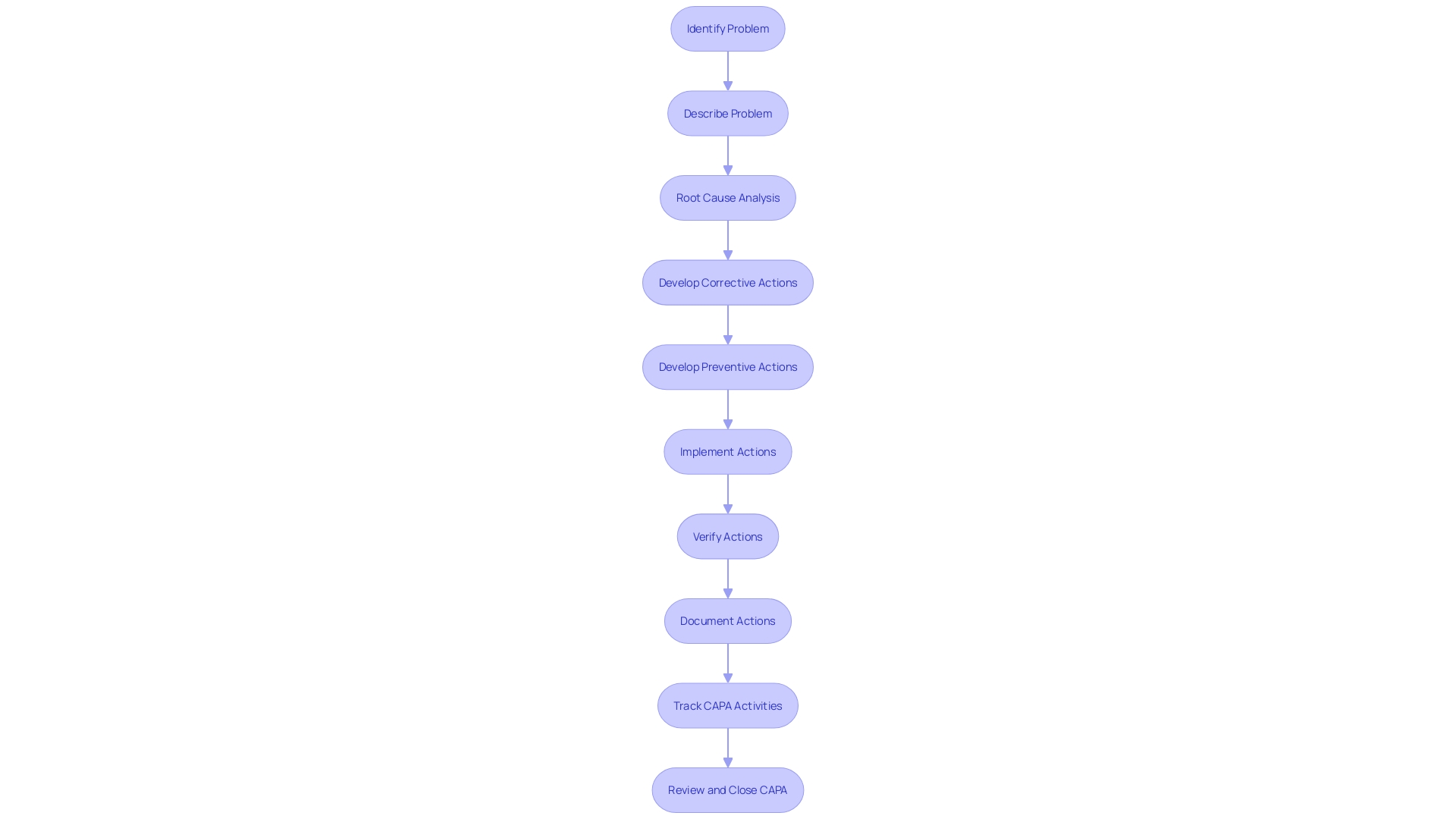
FDA Requirements for CAPA Systems
Producers of healthcare equipment are obliged by the FDA to develop a Corrective and Preventive Action (CAPA) framework, a focal part of the quality management structure. This comprehensive framework is designed to monitor and address product and process issues, ensuring the safety and efficacy of medical devices. The corrective and preventive action process is expected to be thoroughly documented, detailing the problem, root cause analysis, and the actions taken to correct and prevent recurrence. This documentation is vital for transparency and accountability.
An important aspect of the corrective and preventive action (CAPA) system is the training of personnel. Staff must be well-versed in identifying issues, conducting root cause analysis, and implementing the necessary corrective and preventive measures. This training ensures that the team is competent and can effectively manage corrective and preventive action processes.
To confirm the effectiveness of corrective actions, manufacturers must perform checks. These may include analyzing data, testing products, and other methods to confirm that the actions have addressed the identified issues.
If there are any modifications to the medical equipment or its production process resulting from corrective and preventive action (CAPA) endeavors, it is necessary to have rigorous measures in place to control those changes. These changes should be well-documented, evaluated, and validated to maintain the integrity of the device and its manufacturing.
Lastly, corrective and preventive action (CAPA) records must be retained for a period defined by the FDA, allowing for audits and inspections. These records serve as proof of adherence and offer understanding into the efficiency of the corrective and preventive actions process.
Adherence to these FDA requirements is not just regulatory compliance; it is a commitment to quality and patient safety. Rigorous application of these guidelines, including the use of consensus standards, ensures a robust regulatory framework and fosters innovation and standardization in medical device technology.
Essential Elements of an Effective CAPA System
Successfully implementing a Corrective and Preventative Actions (CAPA) framework demands diverse endeavors and rigorous compliance to established protocols. The achievement of such a structure is supported by the steadfast commitment of management. This commitment is not merely vocal; it necessitates the allocation of resources, as well as the provision of comprehensive training and active involvement in CAPA-related activities. Furthermore, the establishment of clear procedures and policies is imperative. These documents are the blueprint for the corrective and preventive action process, detailing the specific responsibilities of personnel, the procedural steps, and the documentation that must be maintained.
The complex nature of corrective and preventive action (CAPA) systems requires smooth cross-functional collaboration. The interaction among different departments is vital, with communication channels requiring to be strong to facilitate the seamless progress of corrective and preventive actions. Furthermore, decisions within the corrective and preventive action framework must be grounded in reliable data. The collection and analysis of accurate data are paramount for pinpointing issues, discerning root causes, and assessing the impact of corrective measures.
A culture of ongoing enhancement is the fertile ground in which a corrective and preventive action mechanism can flourish. This culture utilizes the knowledge acquired from corrective and preventive action processes to improve product quality, streamline manufacturing methods, and optimize overall operations. For example, the Digital Service Team's method of assessing new digital technologies, which involves a comprehensive evaluation of security, compliance, and relevance, demonstrates the careful focus on specifics needed in quality management systems.
Recent progress, like the establishment of UL Solutions' testing laboratory in Michigan, highlights the industry's commitment to innovation and adherence to safety standards. Furthermore, the incorporation of patient and user-centric design principles is becoming more and more impactful in shaping the development of healthcare equipment, highlighting the significance of a corrective and preventive action framework that is adaptable to the ever-changing nature of equipment design and usage. Such initiatives demonstrate the wider industry dedication to improving corrective and preventive action systems, thereby guaranteeing the provision of secure, efficient healthcare equipment to healthcare providers and patients alike.
Common CAPA Problems to Avoid
An effective Corrective and Preventive Action (CAPA) program is crucial in guaranteeing that medical devices not only comply with the necessary standards but also maintain their efficacy and safety throughout their lifecycle. However, specific traps can undermine the integrity of the corrective and preventive action process. One key challenge is the initial step of problem identification. The importance of accurately and comprehensively documenting issues cannot be overstated, as it lays the groundwork for effective corrective measures. The corrective and preventive action system must be equipped with efficient mechanisms for capturing and documenting such issues.
Moreover, the depth of root cause analysis is another critical area. A cursory analysis often leads to inadequate solutions that fail to address the underlying issues. For instance, Medtronic, a global healthcare technology leader, emphasizes the diversity of knowledge and innovative problem-solving approaches to tackle health challenges. This philosophy emphasizes the significance of a comprehensive and careful approach to root cause analysis in the corrective and preventive action process.
When it comes to action development, specificity is key. Corrective and preventive actions must adhere to the SMART criteria – Specific, Measurable, Achievable, Relevant, and Time-bound – to ensure they effectively rectify the identified problems. For example, a study emphasized the need for targeted and actionable interventions, highlighting the necessity of aligning them with identified root causes for successful outcomes.
The implementation and verification stage is equally crucial. Without proper execution and subsequent verification of the actions taken, the entire process of corrective and preventive actions risks being rendered ineffective. Continuous monitoring and data analysis are vital to confirm the resolution of the problem. A case study from an advanced audio-visual experience company in Israel demonstrates the significance of diligent implementation and monitoring in an innovative and risk-prone environment.
Lastly, documentation and tracking are essential to a compliant corrective and preventive action process. Maintaining accurate and complete records is not only necessary for compliance but also for effective monitoring and auditing. As the healthcare equipment sector develops, with companies like Medtronic providing technologies to enhance lives every second, the commitment to strict documentation practices becomes even more relevant.
By comprehending these typical challenges in corrective and preventive actions and gaining insights from prominent figures in the field and real-life scenarios, companies can improve their procedures, thereby supporting the overall objective of providing secure, efficient, and groundbreaking healthcare technologies to patients around the globe.

The Role of CAPA in Quality Management Systems
Corrective and Preventive Measures (CPM) are essential components of the quality management protocols (QMP) for healthcare instruments. They serve as a structured approach for identifying, analyzing, and rectifying issues, thereby ensuring the continuous enhancement of product quality and adherence to regulatory standards.
-
Problem Identification: CAPA mechanisms are intended to systematically uncover and document any discrepancies or non-conformities within medical products or their manufacturing procedures. This is crucial for handling possible issues that may arise from changes in tool design prompted by customer feedback, alterations in materials, or manufacturing processes.
-
Root Cause Analysis: These mechanisms enable a thorough investigation to identify the fundamental causes behind identified problems. For instance, examining the 'three F's'—form, fit, and function—can expose problems associated with a product's components, such as their shape, size, connection, or interaction with other parts.
-
After identifying the underlying reasons, measures are formulated and implemented to correct and prevent issues. Such actions are crucial when changes to a device might necessitate new documentation or regulatory submissions.
-
Through a consistent approach to resolving problems and implementing preventive measures, the continuous improvement process serves as the catalyst for ongoing enhancement of product quality and production methodologies. The collection and management of clinical data from post-market sources, like those facilitated by platforms such as Greenlight Guru Clinical, underscore the importance of continuous oversight in post-market surveillance.
-
Effective corrective and preventive action (CAPA) ensures regulatory compliance and manages risks associated with product quality and patient safety. Adhering to consensus standards, such as those recognized by the FDA and outlined in the Federal Register, is part of this compliance. These standards, developed by Standards Development Organizations (SDOs) based on the principles of transparency, participation, balance, and due process, are instrumental in establishing a robust QMS.
Mary Joyce, vice-president and general manager at UL Solutions mobility and critical group, emphasizes the thriving technology in Michigan, highlighting the state's role in advancing quality through testing and manufacturing excellence. Likewise, Dr. Liz Kwo from Everly Health Solutions emphasizes the potential impact of early disease detection and prevention, which is fundamentally supported by robust quality management and corrective and preventive actions in the healthcare equipment industry.
Best Practices for Implementing and Maintaining CAPA Systems
To improve the efficiency and adherence of Corrective and Preventive Action (CAPA) processes in medical device production, it is crucial to embrace optimal approaches during the execution and continuous oversight phases. A well-defined structure for assigning responsibilities ensures that each team member knows their specific duties, fostering accountability and efficient teamwork. Training is crucial; staff must be adept in identifying problems, conducting root cause analysis, and understanding the action development process along with meticulous documentation.
Data-driven analysis is another foundation of a strong corrective and preventive action approach. By systematically collecting and evaluating data, manufacturers can discern patterns, pinpoint root causes, and measure the effectiveness of corrective actions. Communication also plays a vital role; by fostering open lines of dialogue across departments, companies can facilitate the swift exchange of information, which is instrumental in collaborative problem-solving.
Policies and procedures should not stay unchanged; they need regular reviews and updates to align with the latest regulatory standards and best practices, ensuring the corrective and preventive actions framework remains relevant and effective. Furthermore, conducting periodic internal audits helps identify areas for improvement and verifies regulatory adherence.
Incorporating these best practices into CAPA systems not only drives continuous improvement but also upholds the highest standards of product quality and patient safety. By doing so, medical device manufacturers can not only comply with stringent regulations but also demonstrate their commitment to excellence in healthcare.
Conclusion
In conclusion, the Corrective and Preventive Actions (CAPA) system is a critical component of medical device manufacturing, ensuring product integrity and patient safety. CAPA goes beyond reactive measures and proactively identifies and addresses issues, promoting continuous improvement and adherence to regulatory requirements.
A well-designed CAPA system consists of key components such as problem identification, root cause analysis, formulation of corrective and preventive actions, implementation and verification, and documentation and tracking. These components work together to effectively address issues, prevent recurrence, and ensure continuous improvement.
FDA requirements for CAPA systems include thorough documentation, training of personnel, validation of actions, stringent change control measures, and retention of CAPA records. Adhering to these requirements is not just regulatory compliance, but a commitment to quality and patient safety.
To implement and maintain an effective CAPA system, organizations should allocate resources, provide comprehensive training, establish clear procedures and policies, foster cross-functional collaboration, base decisions on solid data, cultivate a culture of continuous improvement, and adhere to stringent documentation practices.
By following best practices and embracing CAPA systems, medical device manufacturers can enhance compliance, improve product quality, prioritize patient safety, and contribute to the delivery of safe, effective, and innovative medical devices to patients worldwide.




