Introduction
The regulatory landscape for medical devices in Paraguay is shaped by the National Directorate of Drugs and Health Products (DINAVISA), which ensures that all medical devices meet stringent safety and efficacy standards. In an effort to align with international standards, Paraguay's regulatory environment is undergoing significant changes, aiming to streamline processes and facilitate market access for medtech companies. Understanding this framework is essential for navigating the complexities of the Paraguayan market and leveraging the opportunities presented by these regulatory enhancements.
Anticipated regulatory changes in 2024 across various medical fields highlight the need for companies to stay informed and prepared. As the medical device industry evolves, compliance with these regulations is crucial for successful market entry and continued innovation in healthcare.
Regulatory Framework for Medical Devices in Paraguay
The governing structure for medical instruments in the country is managed by the National Directorate of Drugs and Health Products (DINAVISA). This agency is responsible for ensuring that all medical devices meet rigorous safety and efficacy standards through stringent registration, control, and quality assurance processes. 'The oversight environment in Paraguay is undergoing significant changes, with efforts to align with international standards such as those set by the World Health Organization (WHO) and regional guidelines established by the Southern Common Market (MERCOSUR).'}. This alignment aims to streamline compliance processes and facilitate market access for medtech companies. Grasping this framework is vital for companies seeking to maneuver through the intricacies of the Paraguayan market and take advantage of the opportunities offered by these policy improvements. Importantly, considerable legal changes across various medical sectors are expected in 2024, prompting pharmaceutical and medical equipment companies to get ready for these modifications. As the medical equipment industry continues to evolve, manufacturers must stay abreast of these regulatory developments to ensure compliance and successful market entry.
Key Regulatory Bodies and Their Roles
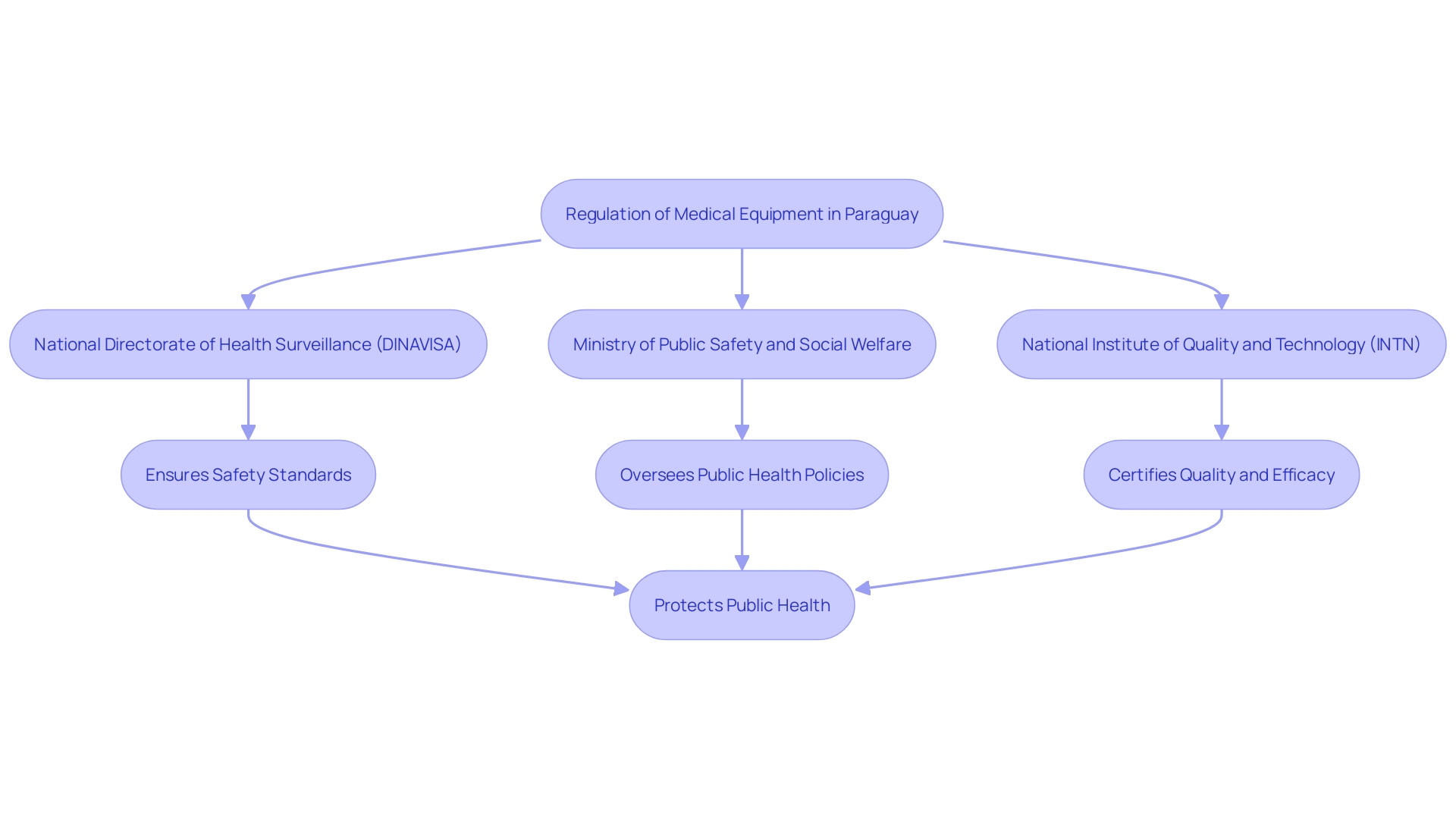
In Paraguay, the regulation of medical equipment is managed by several pivotal bodies, each contributing to a robust oversight framework. The National Directorate of Health Surveillance (DINAVISA) serves as the main authority overseeing wellness products, including medical instruments. Working together with DINAVISA, the Ministry of Public Safety and Social Welfare guarantees extensive public well-being protection measures. Additionally, the National Institute of Quality and Technology (INTN) plays a significant role by providing essential standardization and quality control services. This multi-faceted regulatory framework ensures that medical instruments in the country meet stringent safety and efficacy standards, thereby safeguarding public health.
Classification and Registration Process for Medical Devices
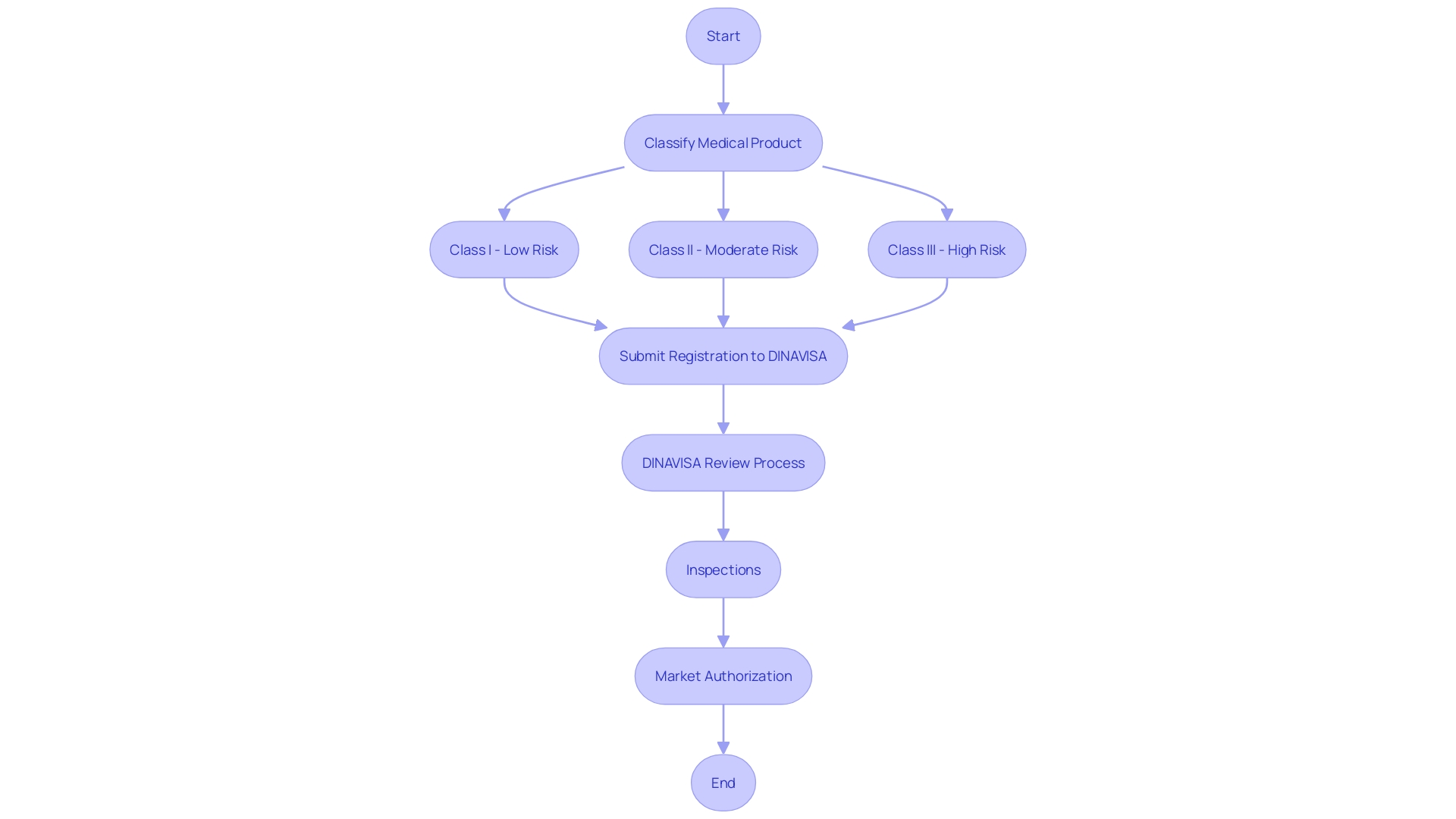
The classification and registration process for medical products in Paraguay is multi-faceted and rigorous, ensuring that only safe and effective items reach the market. Devices are categorized into three classes based on their risk levels: Class I (low risk), Class II (moderate risk), and Class III (high risk). Manufacturers must submit a comprehensive registration application to the National Directorate of Health Surveillance (DINAVISA). This application contains comprehensive documentation on the equipment's safety, efficacy, and quality.
'DINAVISA's review process is thorough and may involve inspections and evaluations by qualified personnel to verify adherence to compliance standards.'. The process is essential in upholding high safety standards and includes the evaluation of scientific, technical, and regulatory information, along with the performance characteristics of the product.
Once the apparatus passes this stringent review, it is granted market authorization, permitting its sale and use within the country. This authorization is crucial for market entry, as it ensures that the product meets all necessary safety and performance criteria, thereby protecting public health and fostering trust in medical technologies.
Clinical Trial Requirements and Procedures
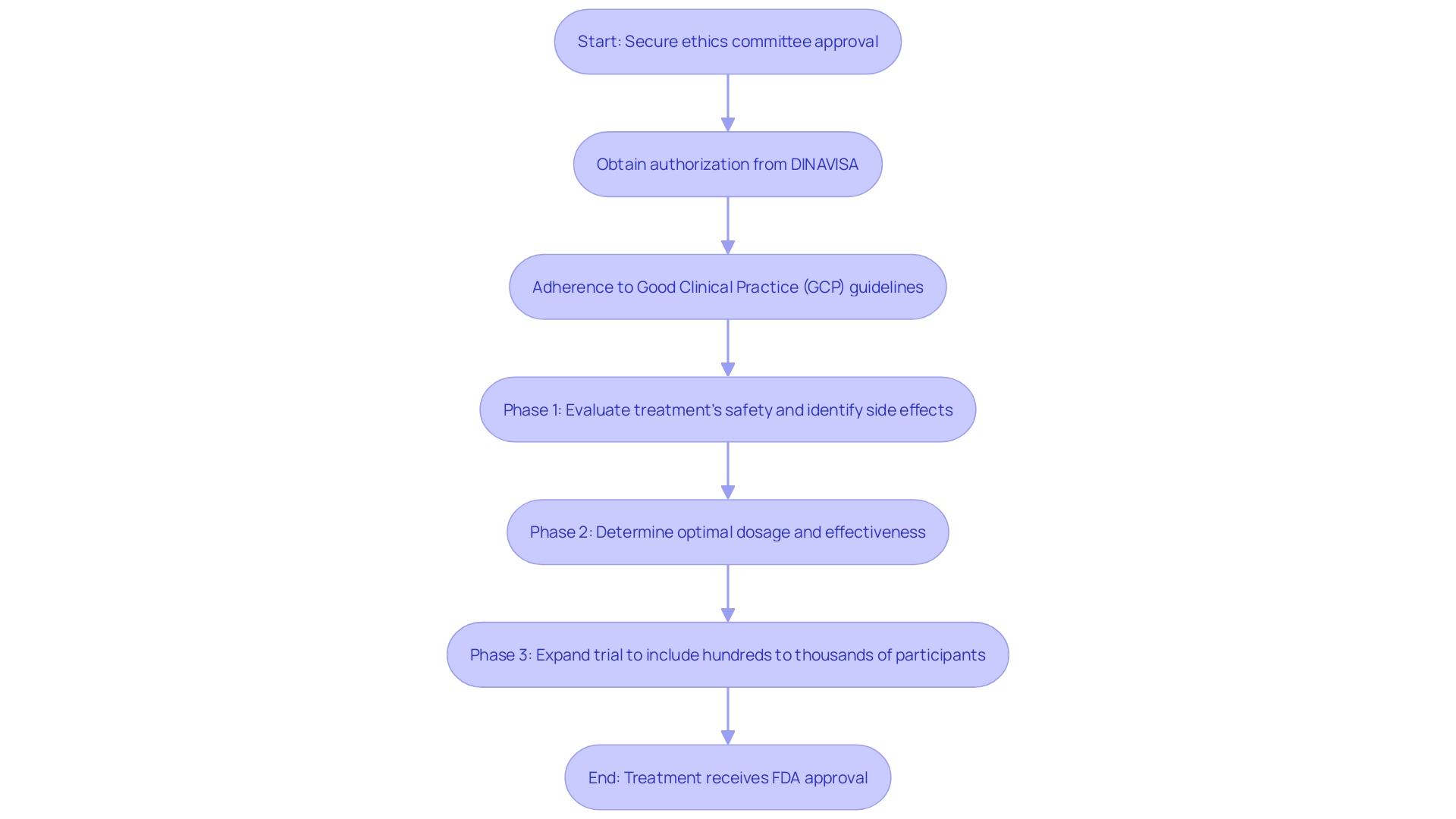
Conducting clinical trials for medical equipment in Paraguay involves a meticulous process to ensure compliance with ethical and regulatory standards. Researchers are required to secure approval from an ethics committee, which rigorously evaluates the trial design to uphold ethical principles. Additionally, authorization from the National Directorate of Health Surveillance (DINAVISA) is essential to commence the trial. This requires the submission of comprehensive documentation, including detailed descriptions of the apparatus, trial protocols, and informed consent procedures. Adherence to Good Clinical Practice (GCP) guidelines is imperative to maintain the integrity of the research and safeguard participant welfare. Given the increasing complexity and costs associated with clinical trials, ensuring compliance with these regulations is crucial for advancing medical innovation.
Barriers and Facilitators for Clinical Research in Paraguay
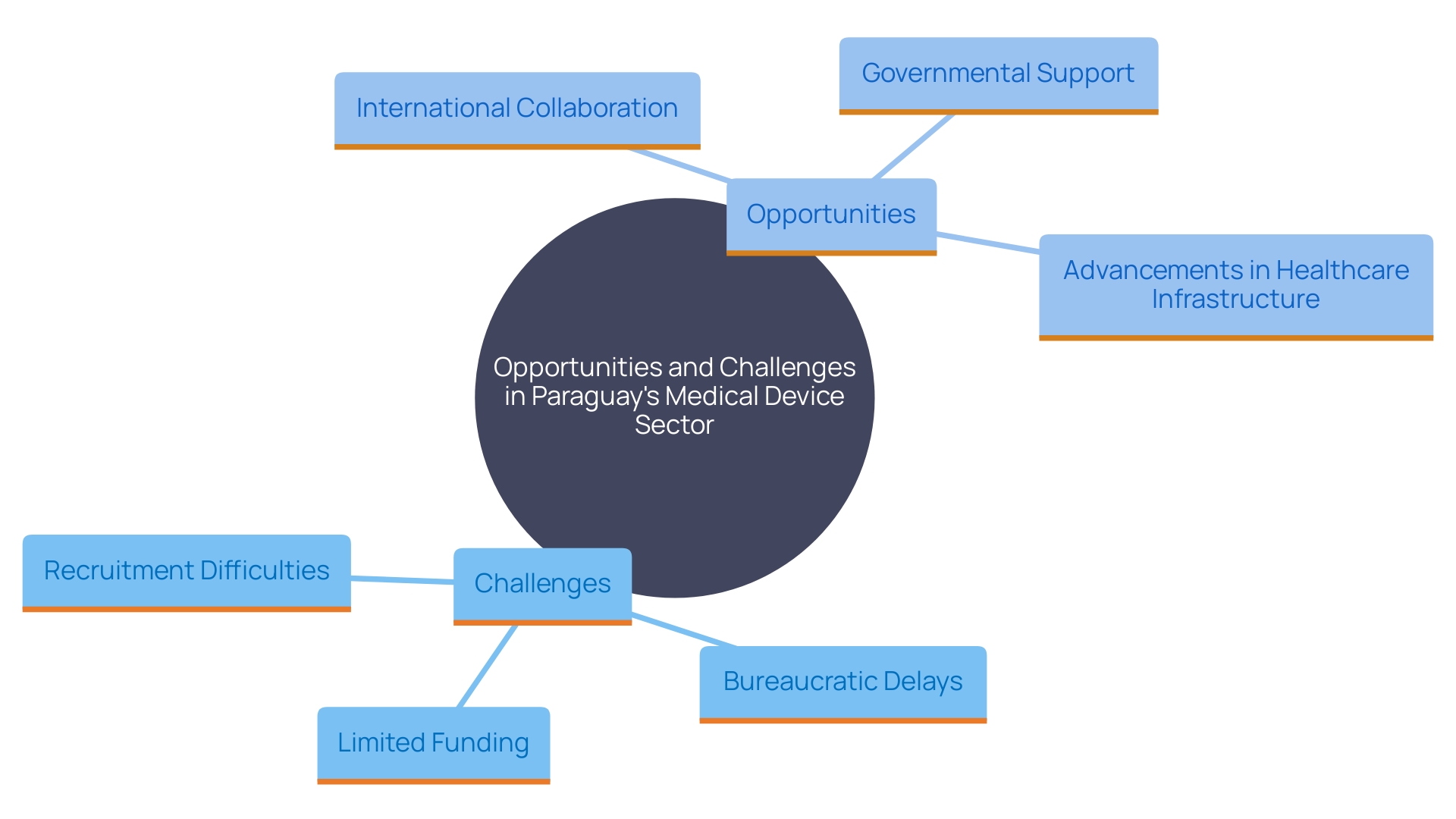
Paraguay presents significant opportunities for clinical research in the medical device sector, but it also faces substantial challenges. Limited funding for research initiatives, bureaucratic delays in regulatory approvals, and difficulties in recruiting qualified personnel are prominent obstacles. Despite these barriers, the country's growing interest in enhancing healthcare infrastructure, collaboration with international partners, and support from governmental organizations present promising facilitators for advancing clinical research.
Tackling these challenges is essential, especially in a context where quality medical systems are vital for community wellbeing and attaining improved wellness results. Approximately 8 million people in low- and middle-income countries (LMICs) die each year from treatable conditions, with 60% of these deaths resulting from poor-quality healthcare. This underscores the importance of effective and equitable healthcare systems.
International collaborations can play a pivotal role in overcoming these challenges. For example, the expansion of telemedicine platforms in Argentina for handling COVID-19 cases emphasizes the potential of digital wellness innovations. In a similar manner, this country can benefit from such innovations to streamline regulatory processes and improve research efficiency.
Real-world examples, such as Brazil's G20 presidency in 2024, illustrate how regional leadership can spotlight health as a central agenda, promoting universal health coverage and sustainability. This global leadership can inspire the nation to adopt similar strategies, ensuring that the country not only addresses its research barriers but also leverages facilitators effectively to advance clinical research in the region.
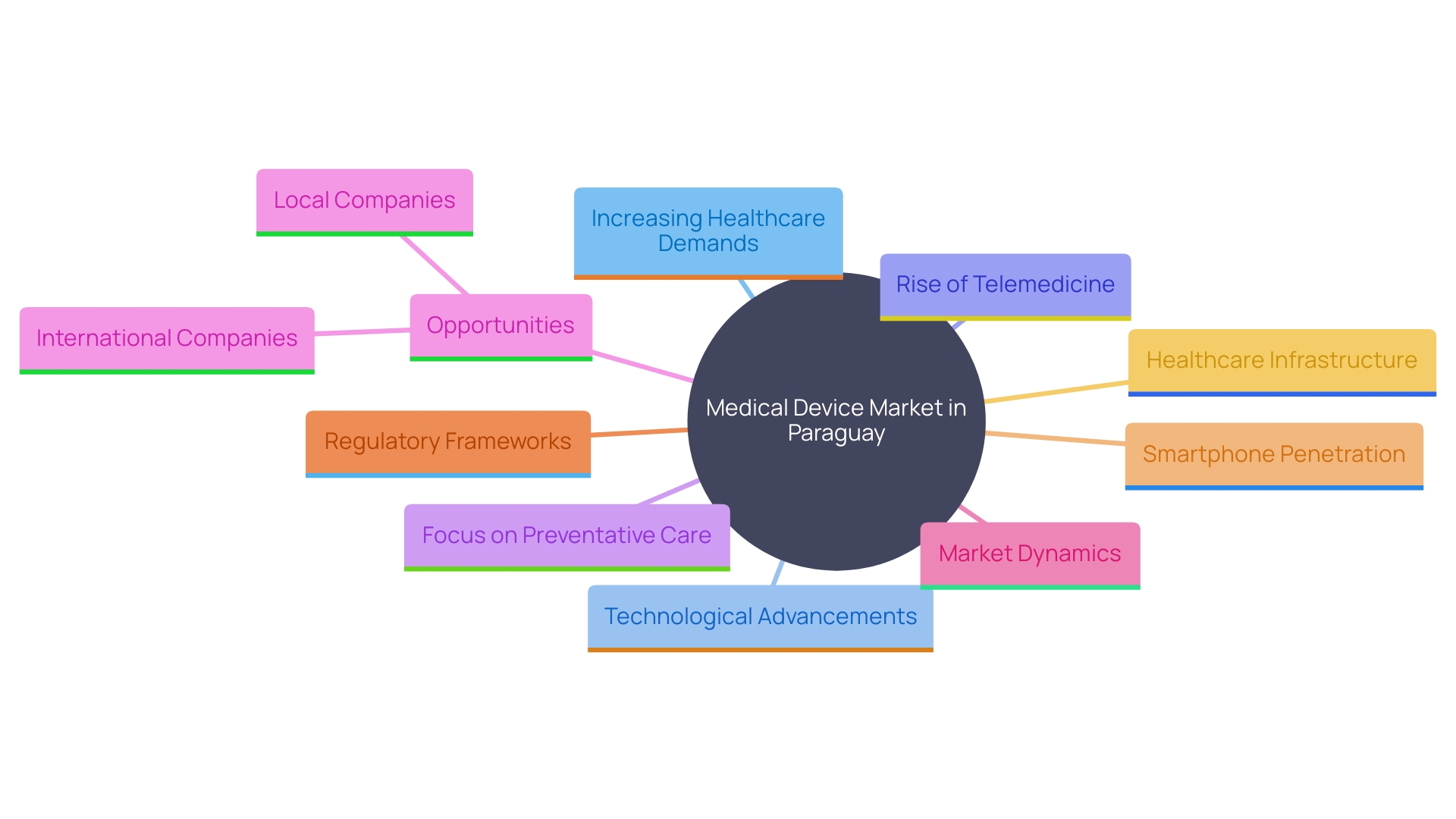
Market Opportunities and Trends for Medical Devices in Paraguay
'The medical device market in the country is burgeoning, propelled by increasing healthcare demands and rapid technological advancements.'. Among the significant trends is the escalating demand for innovative medical technologies, the surge in telemedicine, and a heightened focus on preventative care. This creates abundant opportunities for both local and international companies to introduce new products tailored to address specific health challenges faced by the Paraguayan population. A robust understanding of market dynamics and aligning product offerings with local needs can substantially enhance the potential for success in that country. The market's growth is reflective of broader regional trends, where countries with favorable regulatory frameworks and reimbursement systems for medical devices continue to attract significant investment and innovation. As the nation advances in integrating technology into healthcare, the strong penetration of smartphones and the expansion of telemedicine services are notable developments. These improvements align with the nation's strategic positioning in global connectivity and its commitment to sustainable energy production, further bolstering the healthcare infrastructure.
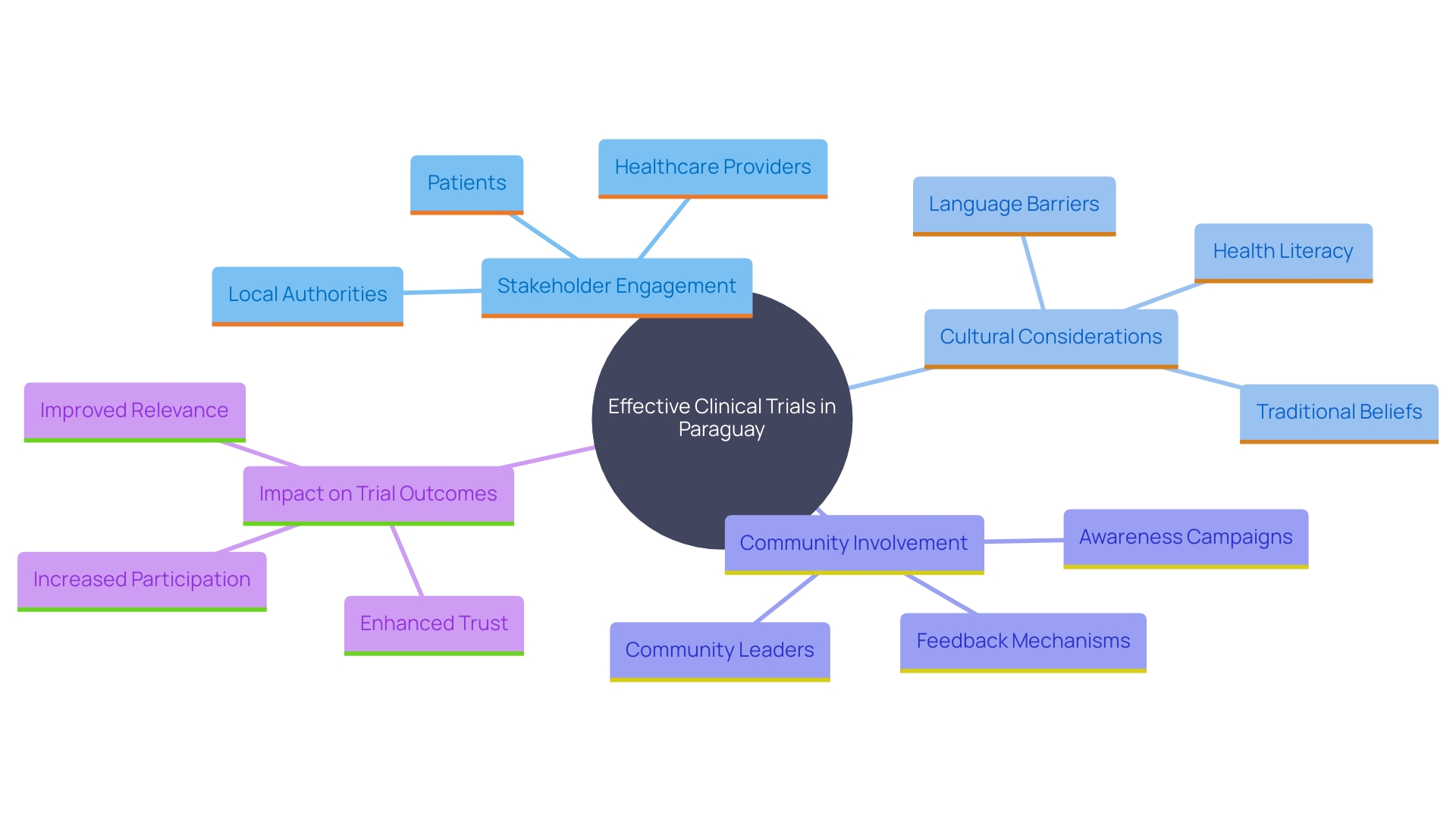
Strategic Considerations for Conducting Clinical Trials in Paraguay
Conducting clinical trials in Paraguay necessitates a strategic approach for optimal results. Engaging with local stakeholders, such as regulatory authorities and healthcare institutions, is crucial to gaining a thorough understanding of the operating environment. Building strong relationships with local investigators is equally important, as it can significantly improve recruitment efforts and streamline trial operations. Considering cultural factors and the characteristics of local patient populations is essential for designing studies that are both relevant and impactful.
The significance of community engagement and involvement in global wellness research cannot be overstated. Involving local communities in research ensures that the studies are undertaken collaboratively with those most affected by the outcomes. This method not only improves the significance of the research but also encourages better well-being results through increased trust and involvement.
'WHO's recent recommendations for national welfare authorities and regulatory bodies emphasize the need for improved trial design, greater diversity of participants, and efficient infrastructure.'. These guidelines aim to address the disparities between high-income and low- and middle-income countries in clinical trials. For instance, in 2022, 27,133 trials were conducted in high-income countries compared to 24,791 in low- and middle-income countries. Enhancing nation-driven research and integrating clinical trials into standard clinical and public welfare services can guarantee quicker and fairer access to medical interventions.
The case of cervical cancer prevention and treatment in Peru serves as an exemplary model of successful collaboration. Training thousands of health workers and transforming disease prevention strategies have had a profound impact on communities. This model highlights the importance of accessibility, education, and empowerment in healthcare. Likewise, strategic partnerships and community involvement in the region can lead to significant improvements in clinical trial outcomes and overall healthcare quality.
In conclusion, a well-thought-out strategy that includes stakeholder engagement, cultural considerations, and community involvement is key to the successful execution of clinical trials in Paraguay. By addressing these factors, researchers can ensure that their studies are effective, inclusive, and beneficial to the local population.
Conclusion
Paraguay's regulatory framework for medical devices is primarily governed by the National Directorate of Drugs and Health Products (DINAVISA), ensuring compliance with stringent safety and efficacy standards. With significant regulatory changes expected in 2024, it is crucial for companies to stay informed and prepared to navigate this evolving landscape to achieve successful market entry.
Key regulatory bodies, including DINAVISA, the Ministry of Public Health and Social Welfare, and the National Institute of Quality and Technology (INTN), collaborate to uphold high safety standards through rigorous classification and registration processes. These efforts are essential for safeguarding public health and fostering trust in medical technologies.
While there are substantial opportunities for clinical research in Paraguay, challenges such as limited funding and bureaucratic delays remain. However, the country's commitment to enhancing healthcare infrastructure and engaging in international collaborations can help address these obstacles. The rising demand for innovative medical technologies and telemedicine further amplifies the market's potential.
To conduct effective clinical trials, it is vital to engage local stakeholders and understand cultural dynamics. Involving communities in research enhances trust and improves health outcomes. By prioritizing these elements, researchers can significantly increase the relevance and impact of their studies.
In summary, Paraguay's dynamic regulatory environment and growing medical device market present both challenges and opportunities. A proactive approach to understanding regulations and engaging local communities will be key to achieving success in this landscape.




