Introduction
Clinical Research Organizations (CROs) are vital players in the development and testing of medical interventions in the pharmaceutical, biotechnology, and medical device industries. These organizations serve as collaborative partners throughout the entire lifecycle of drug and device development, providing specialized expertise in protocol development, regulatory compliance, patient recruitment, and data management. CROs like CMIC Group exemplify the shift from service providers to end-to-end solutions architects, offering comprehensive services that adapt to the evolving requirements of innovative therapies.
The role of CROs extends beyond conducting trials, shaping the future of clinical research by ensuring transparency, research reproducibility, and enhanced patient care. While CROs face challenges such as compliance with regulations and managing stakeholder expectations, they continue to drive medical advancements by embracing specialization, leveraging technology, and globalizing clinical trials. The future of CROs in medical research looks promising, with a focus on participant safety, inclusive research environments, and the pursuit of innovative therapies to improve patient care and expedite medical innovation.
What is a CRO?
Contract Research Organizations (CROs) serve as collaborative partners to the pharmaceutical, biotechnology, and medical device industries, underpinning the development and testing of novel medical interventions. More than just support entities, these organizations are cornerstones in the journey of drug and device development, assisting from the initial conception stage, through preclinical assessments, to the execution and analysis of clinical trials. CROss bring specialized expertise in protocol development, compliance with regulatory standards, patient recruitment strategies, and extensive data management capabilities.
They enable the advancement of medical research by delivering tailored solutions that adapt to the varying requirements of innovative therapies and medical advancements.
CROs, such as CMIC Group, which was a trailblazer in Japan, offer much more than clinical trial management. They provide a comprehensive suite of services that encompass the entire life cycle of pharmaceutical development. CMIC highlights the adaptability and responsiveness of CROss to meet clients' distinct needs at each phase of drug development, reaffirming the vital role these organizations play in the pharmaceutical sector.
CROss like CMIC epitomize the shift from service providers to end-to-end solutions architects for medical innovations and therapeutic advancements.
The depth of CRO engagement in the medical research landscape also encompasses ethical considerations of participant compensation and fair treatment, reflecting a modern understanding that clinical research depends critically on voluntary participation. Insightful perspectives from industry professionals underscore the necessity for strategic planning and decision-making early in the research process. Reflecting on past experiences, experts emphasize the substantial impact that meticulous preparation and comprehensive study design can have on the efficacy and efficiency of clinical trials.
As the demand for transparency in clinical trial reporting increases, the role of CROss extends to ensuring public access to protocols, analysis plans, and data, thereby enhancing research reproducibility and reliability. The integration of CROss in the research process exemplifies the pivotal role they play in not just conducting, but shaping the future of clinical research to better serve patients and the medical community at large.
History of CROs in Medical Research
Clinical Research Organizations (CROs) emerged in the late 1960s as pharmaceutical companies began to outsource aspects of their research to cope with the rising costs and complexities of clinical trials. This trend intensified throughout the 1970s, paving the way for CROss to become a cornerstone in drug development, offering a comprehensive array of research services. Over the decades, CROss have continually adapted to stringent regulations and technological shifts, enhancing their capabilities and accelerating medical research.
In particular, CROs have played a pivotal role by employing innovative approaches such as encapsulating mRNA cargo in lipid nanoparticles marked with CD117 to target hematopoietic stem cells. This cutting-edge technique bypasses the need for ex vivo gene editing, commonly used in treatments for blood disorders like sickle cell disease, which has been both costly and complex.
Moreover, the historical development of CROss echoes the evolution of patent policies critical for medical innovations. Early guidance from Attorney General Tom C. Clark in 1947 about federal employee invention rights suggested a model of government ownership of patents, with march-in rights to ensure inventions are utilized effectively. This set the stage for continuous debate and eventual policies that balance the interests of public welfare with the stimulation of scientific advancement.
Today's CROss continue to be shaped by this heritage, pushing the boundaries of medical research and providing a pathway for new healthcare solutions, illustrated by companies like Freya, which are at the forefront of developing therapies for women's health, addressing the historically unmet medical needs in this field.
Types of CROs
Clinical Research Organizations (CROs) are pivotal in forging pathways towards medical advancements. With their distinct specializations, they provide tailored support to clinical research. Full-service CROs are robust engines that power end-to-end management of clinical trials, right from the nascent study design phase to the final stages of data analysis and reporting.
Specialty Crops, with their laser focus on particular therapeutic areas or defined sectors of research, contribute cutting-edge knowledge and trailblazing expertise that is indispensable for nuanced, specialized trials. Further refining the spectrum are niche CROss, the bespoke artisans of the CRO world, focusing on delivering highly specialized services to particular regions or within unique segments of medical research.
The selection of a CRO is a crucial decision for research sponsors, given the intricate web of regulations, logistics, and the push for efficient, quality outcomes. One patient's journey highlights the complexities involved; envision someone from rural Pennsylvania navigating an international trip to Turkey for a trial—dealing with visas, language barriers, and transport—where a CRO's expertise in global trials can prove invaluable.
Indeed, the integrity of data in clinical trials is paramount, and CROs are instrumental in ensuring accurate, high-quality data collection. In a landscape where a single missing data point can jeopardize an entire study's credibility, specialty CROs demonstrate their worth by meticulously managing data integrity, particularly when dealing with sensitive patient-reported outcomes.
Moreover, the dynamics between pharmaceutical companies and CROs are continually evolving. Recognizing the need for more effective clinical trial processes, a biotech clinical program director emphasized the necessity for CROss while also acknowledging the challenges inherent in the collaboration due to the scale and structure of these large organizations.
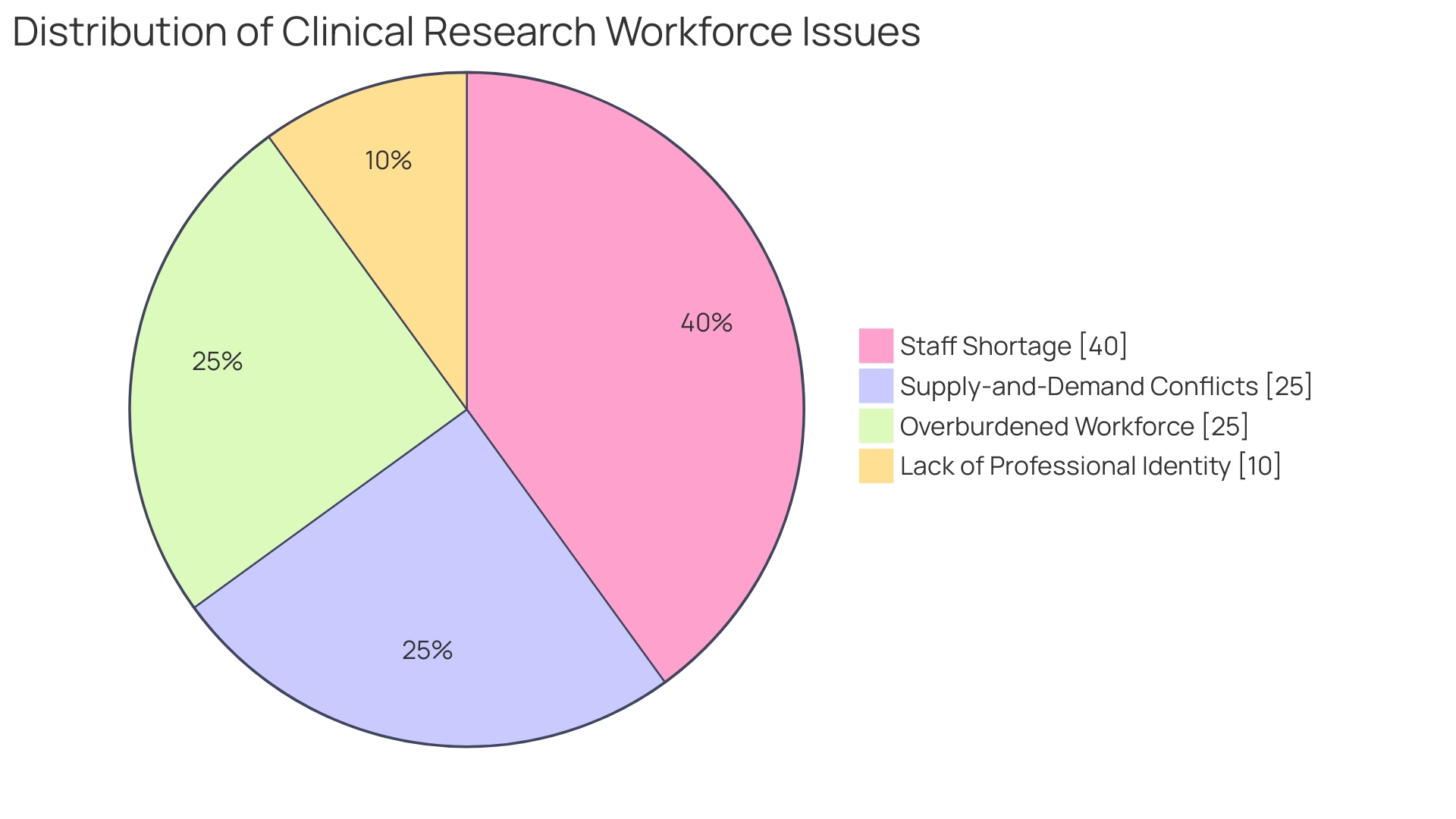
To encapsulate the gravity of CROs in the clinical trial ecosystem, consider the remarks from a biomedical engineer with extensive experience managing pivotal clinical studies: his role amplifies the critical position CROss hold not just in facilitating research but in shaping the future of healthcare delivery. With a dire shortage within the clinical research workforce—where available positions outnumber experienced applicants—the CROss role becomes even more pronounced, as they bring expertise and stability to the increasingly demanding world of clinical research.
Role of CROs in Preclinical Research
Contract Research Organizations (CROs) are at the forefront of preclinical research, which is the foundation upon which new pharmaceuticals and medical devices are built. The preclinical phase is vital for assessing the safety and efficacy profile of new therapeutic candidates before they make their way to human clinical trials. Specializing in the intricate process of preclinical experimentation, CROs are the crucial partners that pharmaceutical and biotechnology companies rely on to fill their scientific and infrastructural gaps.
During preclinical research, CROs undertake a rigorous regimen involving in vitro testing and animal studies, meticulously designing each experiment to meet both scientific and regulatory standards. Their expertise ensures that data collected are robust and comprehensive, forming the bedrock of evidence that decision-makers rely on to forge ahead in the highly competitive realm of drug development. The process itself is an arduous journey spanning years and, more often than not, incurs monumental costs, which may reach up to $2.6 billion.
In recent times, advances in AI, such as Gas and the innovative GENTRL model, have been leveraged to enhance preclinical research, thus catalyzing the drug discovery process. Real-world impacts of these CRO-led studies are vast, extending beyond the research domain; they influence treatment procedures and improve survival rates. For instance, the limited initial treatment options for neuroblastoma—a cancer underscored as 'peculiar'—have seen expanded paths for care, thanks in part to adaptive and evolving clinical research.
Moreover, each successful step in preclinical research boosts the pursuit of effective treatments and aids in the identification of potential therapies. As highlighted by the poignant stories shared by patients and the CRO's commitment to providing a positive research experience, clinical research participants contribute immensely to medical progress that can benefit countless individuals around the globe.
CROs not only spearhead the complex phases of drug discovery but also serve as a focal point in the clinical research ecosystem. Their scalable scientific programs, as experts suggest, aim to augment laboratory research; however, ultimate accountability for results rests with the principal investigators overseeing the projects.
Nonetheless, the growth and demand for skilled clinical research professionals far exceed available talent, challenging CROs and the industry at large. The high attrition rates among personnel accentuate a crisis in the workforce, further underlining the urgency of robust and proactive recruitment strategies to advance and sustain the vital research orchestrated by these organizations.
Role of CROs in Clinical Research
Clinical Research Organizations (CROs) fulfill an integral role within the fabric of healthcare innovation, bridging the chasm between laboratory research and the practical, life-saving treatments made available to patients. These entities are essential in administrating the labyrinthine process of clinical trials, collaborating with a litany of partners including sponsors, healthcare professionals, and regulatory bodies to ensure that from inception to completion, each trial adheres to stringent protocols and regulations.
Engaging in an assortment of crucial functions, CROs streamline the complex ecosystem of clinical trials by adeptly managing logistics; from discerning suitable research sites to enlisting and monitoring participants, these organizations facilitate every phase. As the conduits of data management and quality assurance, CROss ensure that the integrity of data drives sound conclusions and propels medical advancements forward.
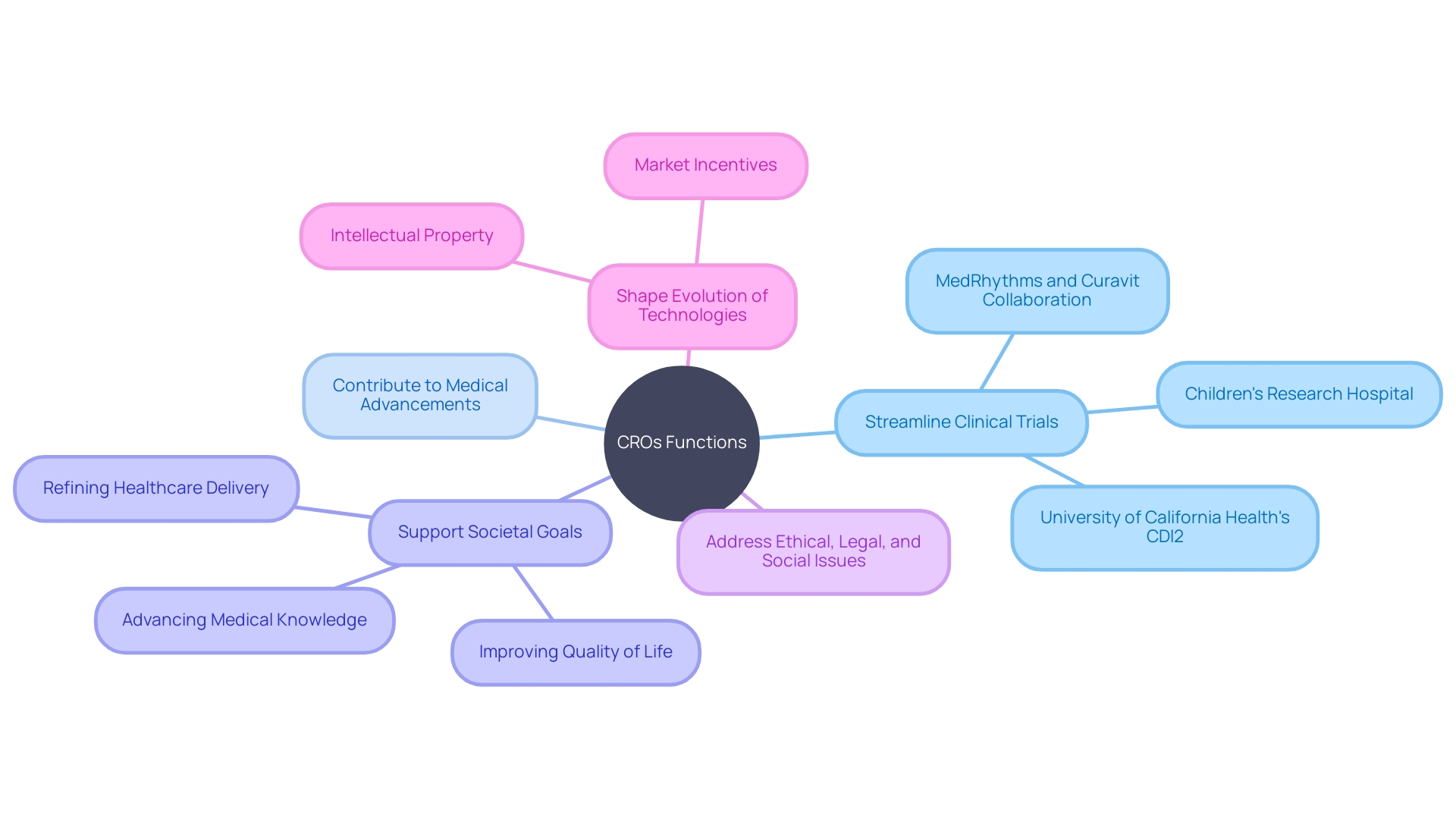
One such beacon of progress exemplified through clinical research is Children's Research Hospital, which not only epitomizes excellence in pediatric cancer treatment but also in clinical trial management. Emphasis on generating high-caliber data through trials has translated into quantum leaps in survival rates for childhood cancers, echoing the indispensable role of thorough and well-executed clinical research.
A poignant example of CRO impact is reflected in the advent of cutting-edge treatments for diabetes and obesity being observed by University of California Health's Center for Data-driven Insights and Innovation (CDI2). Laying foundation to innovative frameworks, CDI2's incorporation of real-world clinical data to evaluate treatment efficiencies heralds a paradigm where the rigor of CRO-led research synergizes with substantive data analytics, yielding accelerated medical discoveries and optimizing patient-centric approaches.
In keeping with this innovative spirit, anticipates the overarching global benefits of its discoveries, democratizing the advancements by sharing breakthroughs for the worldwide medical community to implement. Additionally, MedRhythms and Curavit's collaboration on the Orchestras trial exemplifies the monetary wisdom of melding novel healthcare technologies with meticulous research oversight, ensuring not only breakthroughs in patient outcomes but also sustainable economic impacts.
The elaborate landscape of CRO services and their consequential outcomes reiterates that the vitality of clinical research extends beyond the immediate realm of developing new therapies. It underpins larger societal goals like advancing medical knowledge, refining healthcare delivery, and enhancing the quality of life across diverse populations—goals that CROss are uniquely positioned and equipped to fulfill.
Advantages of Using CROs
Clinical Research Organizations (CROs) are pivotal in shaping the landscape of medical research, and their benefits extend far across the realm of healthcare innovation. With the unparalleled specialization and expertise that CROss offer, they bolster the efficiency and integrity of clinical trials. For instance, the University of California Health's Center for Data-driven Insights and Innovation has leveraged actual clinical data to considerably mitigate research costs and expedite the discovery of efficacious medications.
This paradigm of innovation not only saves time for healthcare professionals but also enhances the patient care experience by hastening the journey towards effective treatment options.
Furthermore, CROs act as crucial conduits to a widespread network of research sites and investigators, elevating patient recruitment efforts and magnifying the scope of studies. Access to cutting-edge facilities and technologies via CROss translates to heightened data precision and substantial progress in clinical research outcomes. CROs offer an ecosystem where state-of-the-art technology and data analysis converge, as indicated by a study in the Journal of the American Medical Association, which underscores the transformative potential of employing real-world clinical data across different health centers.
The deployment of CROs in the research spectrum not only streamlines operational aspects but also presents financial pragmatism. A survey conducted by CRO Worldwide Clinical Trials revealed an industry leaning towards midsize CROs, valuing their agility, personalized attention, and senior-level expertise—a testament to the evolving preferences and requirements in medical research partnerships.
Lastly, the role of artificial intelligence and machine learning within the CRO framework cannot be overstated. They mark the advent of an era where precision and personalization in patient treatment are paramount, as iterated by Gary Shorter, Head of Artificial Intelligence at IQVIA Technologies. The foresight to tap into advanced technology systems and digital workflows is key in manifesting an efficient and patient-centered future for clinical trials.
Challenges Faced by CROs
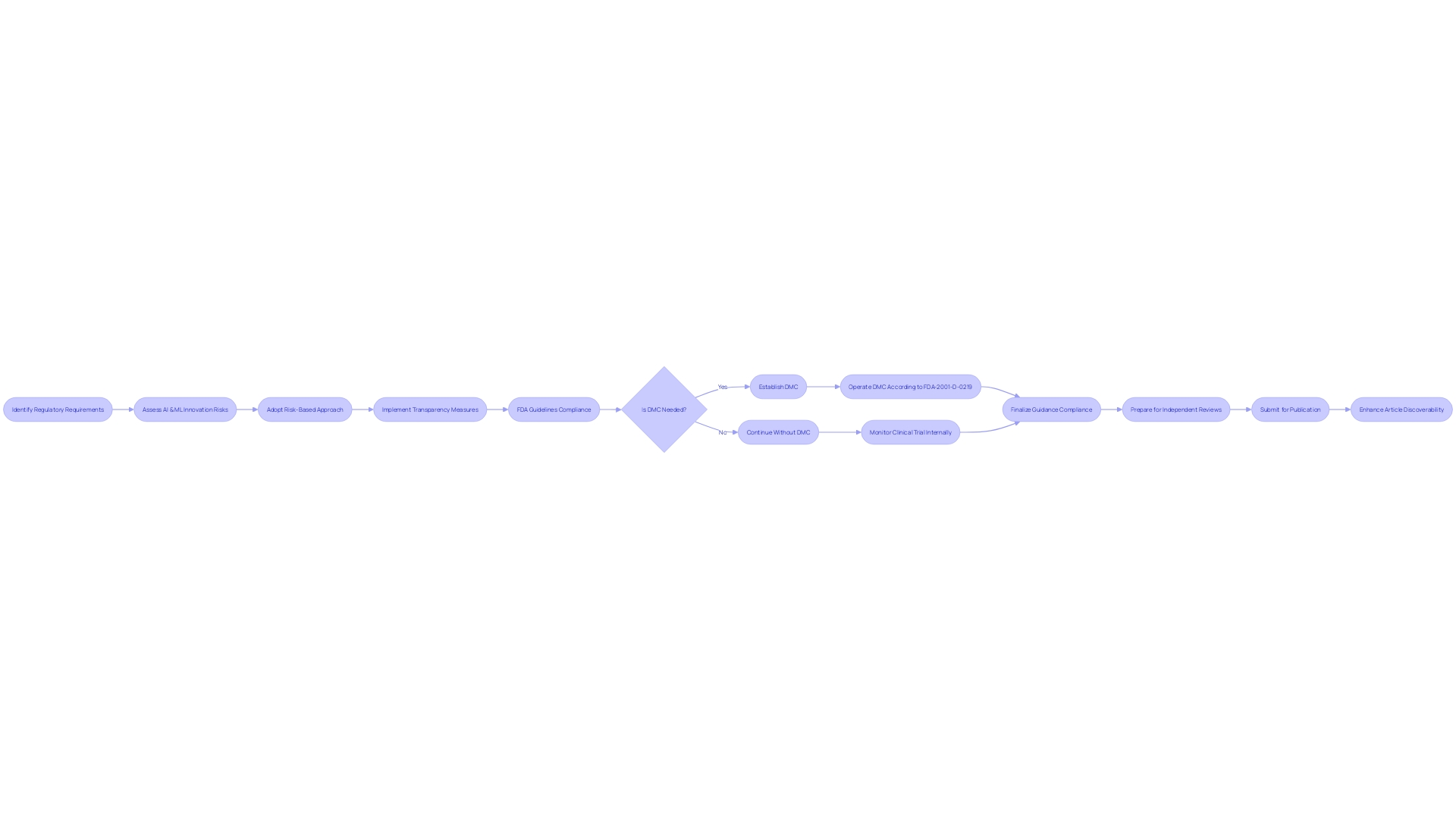
Clinical Research Organizations (CROs) are vital in shaping the future of medicine by ensuring that clinical trials are conducted with the highest standards of safety and efficacy. Their task is no easy feat; CROss grapple with a complex array of regulatory guidelines that can vary significantly by region, including those laid out by the FDA and EMA. As technology in medical research progresses, CROs incorporate advancements such as AI and ML, always with a vigilant eye on the accompanying compliance obligations.
Regulations such as the EU AI Act require a risk-based approach to deploying these technologies, highlighting the need for transparency and patient safety.
Patient-centric drug development is taking precedence in the industry, spurred by the accessibility of data through connected devices and decentralized trial solutions. The integration of traditional trial components with digital data sources necessitates a robust data strategy before the trial protocol is even set. This strategy must address how data will be collected, monitored, cleaned, and analyzed to extract actionable insights while ensuring patient safety and data integrity.
Clinical trial success relies not only on technical compliance but on managing stakeholder expectations and mitigating potential burdens, as raised in the questions about data management and flow strategy. For example, participants need answers about the logistics of cross-border travel to join a study, and these practicalities are part of the trial design. Moreover, data governance reforms, as per the recent draft update on the European GCP guidance, aim to safeguard participants' rights, their safety, and the reliability of trial results.
The reframed industry view goes beyond mere technology adoption. It encompasses a fundamental shift in clinical trial methodologies—a shift clearly articulated by Silvia Perez at OCT Europe 2024. There, she underscored the four key processes of data governance aimed at protecting participants and informing key decision-making, positing that the true goal of clinical trials should be the protection of patient rights and ensuring trial integrity.
What transpires from these regulatory shifts and technological innovations is a continuous effort by CROss to harmonize practices on an international scale and remain agile amidst the ever-evolving medical research landscape.
Future of CROs in Medical Research
Clinical Research Organizations (CROs) are expanding their horizons, with industry experts predicting a colorful future for these pivotal players in medical advancements. Specialization is one trend steering the market, where CROss with niche expertise, such as rare disease research or specific therapeutic areas, are in high demand. Chris, an experienced biomedical engineer, contributes to this precision by applying his extensive background in managing clinical studies for advanced medical devices to enhance CRO functions.
In parallel, CROs are harnessing technology to break new ground in clinical research. AI and machine learning emerge as transformative forces, refining everything from patient recruitment to data analysis. This technological shift not only augments the efficiency of research but also poses opportunities for trial emulation, where existing data can echo the benefits of potential treatments without extensive trials.
Furthermore, clinical trials are no longer bound by geography; globalization enables CROs to tap into diverse and remote participant pools, deftly handling complex logistical challenges that come with international studies.
As we navigate our post-pandemic reality, the reshaping of conventional clinical trial models is evident. This evolution is underpinned by a commitment to participant safety and an inclusive research environment. The industry's agility, particularly among midsize CROs, is lauded for offering intimate scientific collaboration and rapid response capabilities, addressing sponsor needs more effectively compared to the 'one-stop-shop' approach of larger counterparts.
Tailored services, tech integration, and global outreach cement the indispensable role of CROs in nurturing novel therapies and delivering insights into the treatment of maladies that affect millions globally. Their strategic operations stand as a testament to the undying quest to elevate patient care and expedite medical innovation.
Conclusion
In conclusion, Clinical Research Organizations (CROs) are vital players in the development and testing of medical interventions. They provide specialized expertise throughout the entire lifecycle of drug and device development, adapting to the evolving requirements of innovative therapies. CROs ensure transparency, research reproducibility, and enhanced patient care.
CROs have a rich history of adapting to regulations and shaping patent policies for scientific progress. They come in different types, offering tailored support and expertise to clinical research. The demand for skilled clinical research professionals highlights the crucial role of CROs in sustaining and advancing medical research.
In preclinical research, CROs fill scientific and infrastructural gaps, conducting rigorous experiments and collecting robust data. Advances in AI and machine learning enhance preclinical research, contributing to the development of effective treatments.
In clinical research, CROs facilitate logistics, data management, and quality assurance. They collaborate with multiple partners to ensure adherence to protocols and regulations. CROs have been instrumental in fields like pediatric cancer treatment, diabetes, and obesity, leading to significant advancements and improved patient outcomes.
The advantages of utilizing CROs include enhanced efficiency, access to a wider network of research sites, and integration of cutting-edge technologies and data analysis. The role of AI and machine learning is crucial in realizing personalized patient treatment. CROs also face challenges such as compliance with regulations, managing stakeholder expectations, and ensuring patient safety and data integrity.
The future of CROs looks promising, with specialization, technological advancements, and globalization playing significant roles. CROs with niche expertise are in high demand. The implementation of AI and machine learning is transforming the research landscape.
The industry's commitment to participant safety and an inclusive research environment, as well as the emphasis on tailored services and global outreach, solidify the indispensable role of CROs in advancing patient care and medical innovation.




