Introduction
Latin America has emerged as a compelling destination for Medtech clinical trials, offering a dynamic environment marked by diverse patient populations, favorable regulatory frameworks, and cost-effective operations. The region's demographic variability provides invaluable data across various ethnic and socioeconomic groups, enhancing the generalizability of clinical outcomes, particularly for diseases influenced by genetic factors such as cancer. Countries like Mexico, Brazil, and Colombia have streamlined regulatory processes to attract foreign investments, creating a more efficient landscape for initiating clinical trials.
Furthermore, the cost advantages and growing focus on healthcare advancements position Latin America as a strategic hub for Medtech research, evidenced by significant investments from leading companies in the region. This article delves into the factors that make Latin America an attractive destination for Medtech clinical trials, the key players in the region's CRO sector, the benefits of conducting trials in this locale, the challenges faced, and the technological advancements that are reshaping the healthcare landscape.
Why Latin America is an Attractive Destination for Medtech Clinical Trials
Latin America provides a dynamic setting for Medtech research trials, marked by diverse patient populations, regulatory environments, and cost-effective execution. The demographic variability in this region is invaluable for collecting comprehensive data across various ethnic and socioeconomic groups, ensuring the applicability of health outcomes. For example, promoting diversity in medical research is essential, as different populations may show varying responses to treatments; this is particularly important for diseases like cancer, where genetic factors can affect the effectiveness of therapies.
'Numerous American nations, including Mexico, Brazil, and Colombia, have made significant progress in streamlining their regulatory processes to attract foreign investments and innovations in healthcare.'. These efforts have created a more favorable environment for initiating clinical studies efficiently. The emphasis on policy prioritization and cost-effective innovation ensures that the costs of diagnosis, treatment, and care do not become barriers to advancements in medical research and patient care.
'Cost-effectiveness is another significant advantage of conducting trials in South America.'. Lower operational costs, coupled with a growing focus on healthcare advancements, position the region as a strategic hub for Medtech research. Companies like Freudenberg Medical and Trelleborg Healthcare & Medical are expanding their operations in Costa Rica, highlighting the region's potential for supporting complex manufacturing and assembly processes. Freudenberg Medical, for example, is investing $25 million in a new facility dedicated to assembling high-precision medical devices, which will become operational in 2025. This expansion highlights the strategic significance of America in global Medtech research and development.
Key Players in Latin America's CRO Sector
The CRO sector in Latin America is a dynamic and evolving landscape, marked by a blend of local and international players. Major global CROs have established a strong presence in the region, leveraging the local expertise necessary to navigate complex regulatory environments and cultural nuances effectively. A prime example is Everest Clinical Research, a full-service CRO that has earned a reputation for high-quality deliverables and superior customer service across the pharmaceutical, biotechnology, and medical device industries. These global entities work alongside emerging local CROss, which are gaining prominence by offering specialized services tailored to Medtech innovations. This partnership cultivates a strong environment, improving the productivity and efficacy of research studies.
Furthermore, the region's CROs are pivotal in forming strategic partnerships with hospitals, research institutions, and regulatory bodies. These alliances are crucial in driving advancements in healthcare innovation. For example, Fortrea's method for research solutions emphasizes the significance of flexibility and quickness in study design, development, and delivery. By providing personalized testing solutions through comprehensive service, functional service providers, or hybrid models, Fortrea illustrates the customized approach required to satisfy varied client needs.
The strategic planning and competitive intelligence provided by these CROss offer clear insights into opportunities and risks, ensuring that stakeholders can make informed decisions. This comprehensive support system is vital for the successful navigation of the research environment in South America, ultimately contributing to improved patient outcomes and advancements in medical knowledge.
Benefits of Conducting Clinical Trials in Latin America
Carrying out medical studies in South America offers numerous attractive benefits, including faster participant enrollment, reduced expenses, and entry to a growing market. The region's medical infrastructure has seen significant investments, with countries bolstering healthcare facilities and enhancing training for medical professionals. This progress creates a robust environment for clinical research, facilitating faster trial timelines. The increasing prevalence of chronic diseases in the region offers a substantial patient pool, making it easier for sponsors to meet enrollment targets and accelerate the development of Medtech innovations.
Brazil's forthcoming G20 leadership in 2024 highlights the increasing significance of America in the worldwide wellness arena. The nation, prepared to guide the G20 for the first occasion, has a remarkable chance to showcase regional wellness leadership and tackle international public well-being challenges. The lively and varied medical and scientific skills of the region are gaining more acknowledgment, aiding progress in well-being equality and medical innovation.
Furthermore, the Quality Evidence for The Transformation of Health Systems for Latin America and the Caribbean (QuEST LAC) network exemplifies the region's commitment to improving healthcare quality. By uniting researchers from nations such as Peru, Argentina, Ecuador, Mexico, and Colombia, Quest LAC seeks to enhance human capacity in systems research and encourage high-quality medical practices. This collaborative effort is crucial, as it addresses the disproportionate impact of poor-quality healthcare on disadvantaged populations.
To enhance research outcomes, sponsors are utilizing advanced data and analytics methods for site selection and management. By leveraging rich data sets, electronic medical records, and AI-driven models, sponsors can identify opportunities to accelerate recruitment by 15 to 20 percent, ensuring faster and more efficient clinical trials. This strategic approach is vital in a region where the evolving healthcare landscape demands innovative solutions to meet growing public health needs.
Challenges and Solutions for CROs in Latin America
CROss in the region of America encounter several significant challenges, including regulatory discrepancies, varying healthcare standards, and logistical hurdles. Each country in the region operates under its own unique set of regulations, complicating multi-country studies. To navigate these complexities, CROss must take a proactive approach by closely collaborating with local regulatory authorities to ensure compliance. For example, Brazil's National Health Surveillance Agency (ANVISA) has faced issues with its controlled substances database since 2021, highlighting the importance of staying updated with local regulatory environments. Solutions to these challenges include investing in local talent who possess an in-depth understanding of the regional regulatory landscape and fostering robust relationships with healthcare providers, which can enhance operational efficiency. Furthermore, data flow management continues to be an essential element, as contemporary trials frequently combine various data sources, necessitating careful planning and coordination. By addressing these factors, CROs can better manage the complexities of conducting medical research in South America.
Technological Advancements in Latin America's Healthcare Sector
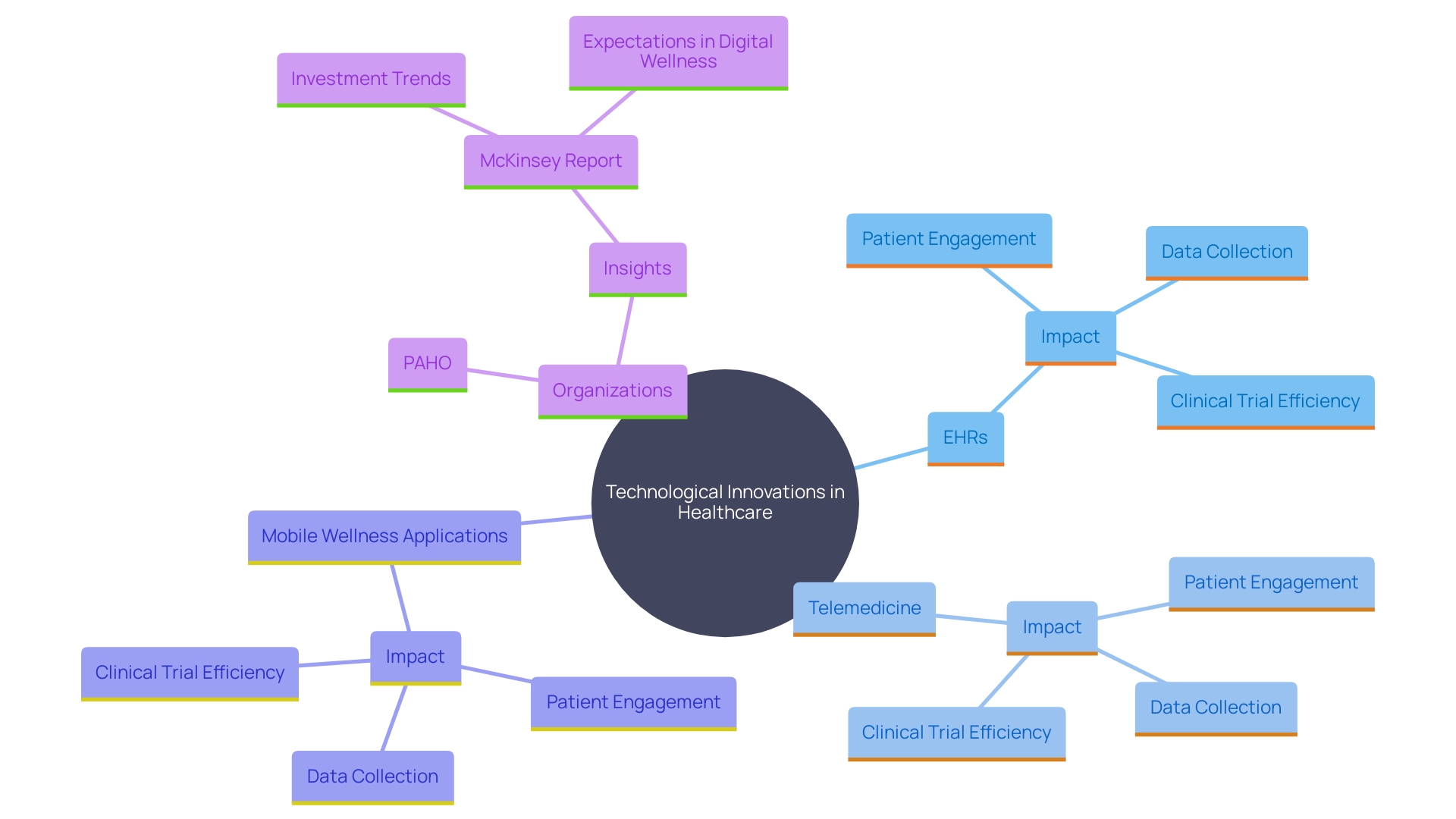
Technological advancements are reshaping healthcare in Latin America, providing innovative tools and methods for clinical research. 'The integration of electronic medical records (EHRs), telemedicine, and mobile wellness applications is revolutionizing patient engagement and data collection.'. These innovations streamline study processes, enhance patient monitoring, and improve follow-up, resulting in more reliable outcomes.
Maria Celeste Savignano, Chief of the Telehealth and Innovation Department at Garrahan Hospital in Argentina, emphasized the transformative impact of telemedicine in specialized care. The Pan American Health Organization (PAHO) has also been instrumental in advancing digital wellness through initiatives like the All in One Telemedicine Package. This package includes a customizable telemedicine platform, training resources for medical personnel, and a telehealth kit with high-quality medical digital devices, tailored for diverse settings.
The potential of digital wellness is further emphasized by a McKinsey report, which highlights the substantial impact anticipated from virtual care and digital entry points, with 70 percent of system executives expecting high advantages. However, there is a notable gap in investment in AI, with 88 percent acknowledging its high potential impact, yet only 20 percent planning to invest in the next two years.
As the healthcare sector evolves, CROss adopting these technologies will be better positioned to conduct efficient and effective clinical trials, ultimately contributing to improved health outcomes in the region.
Conclusion
Latin America stands out as a pivotal region for Medtech clinical trials, offering a unique combination of diverse patient populations, streamlined regulatory frameworks, and cost advantages. The demographic variability inherent in the region allows for comprehensive data collection that enhances the generalizability of clinical outcomes. This aspect is particularly crucial for conditions influenced by genetic factors, such as cancer, and underscores the importance of diversity in clinical research.
Countries like Mexico, Brazil, and Colombia have made significant strides in refining their regulatory processes, facilitating a more efficient environment for clinical trials. The focus on cost-effective operations further positions Latin America as a strategic hub for Medtech research, as evidenced by substantial investments from leading companies. The presence of prominent global and local CROs fosters collaboration that enhances the efficiency and effectiveness of clinical trials, ultimately driving advancements in healthcare innovation.
While the advantages are compelling, challenges such as regulatory discrepancies and logistical hurdles must be addressed. Proactive collaboration with local authorities and investment in local talent are essential strategies for overcoming these barriers. Moreover, technological advancements, including the integration of electronic health records and telemedicine, are reshaping the healthcare landscape, enabling more effective patient engagement and data collection.
In summary, Latin America's potential as a destination for Medtech clinical trials is underscored by its diverse patient demographics, evolving regulatory landscape, and cost-effectiveness. By leveraging these strengths while addressing existing challenges, stakeholders can unlock significant opportunities for innovation and improved health outcomes in the region.




