Introduction
The FDA plays a crucial role in ensuring the safety and effectiveness of medical devices before they can be marketed in the United States. Understanding the FDA's regulatory pathways, such as approval and clearance, is essential for industry professionals and healthcare providers. This article provides an overview of these pathways and their distinctions, highlighting the rigorous evaluation processes that medical devices undergo.
By delving into the FDA's commitment to public safety and its recent initiatives to enhance regulatory efficiency, this article aims to shed light on the importance of navigating the FDA's regulatory landscape accurately. Whether it's the Pre-Market Approval (PMA) process for high-risk devices or the 510(k) clearance process for lower-risk devices, each pathway requires specific evidence to demonstrate a device's safety and effectiveness. By understanding these distinctions, industry professionals can streamline the regulatory process and bring innovative medical devices to market more efficiently.
Moreover, this article emphasizes the implications of FDA approval and clearance for both manufacturers and consumers, highlighting the significance of these regulatory milestones in ensuring patient safety and instilling confidence in medical devices. Stay tuned to grasp the nuances of FDA regulations and standards, essential for the successful development and marketing of medical devices.
Understanding FDA Approval and Clearance
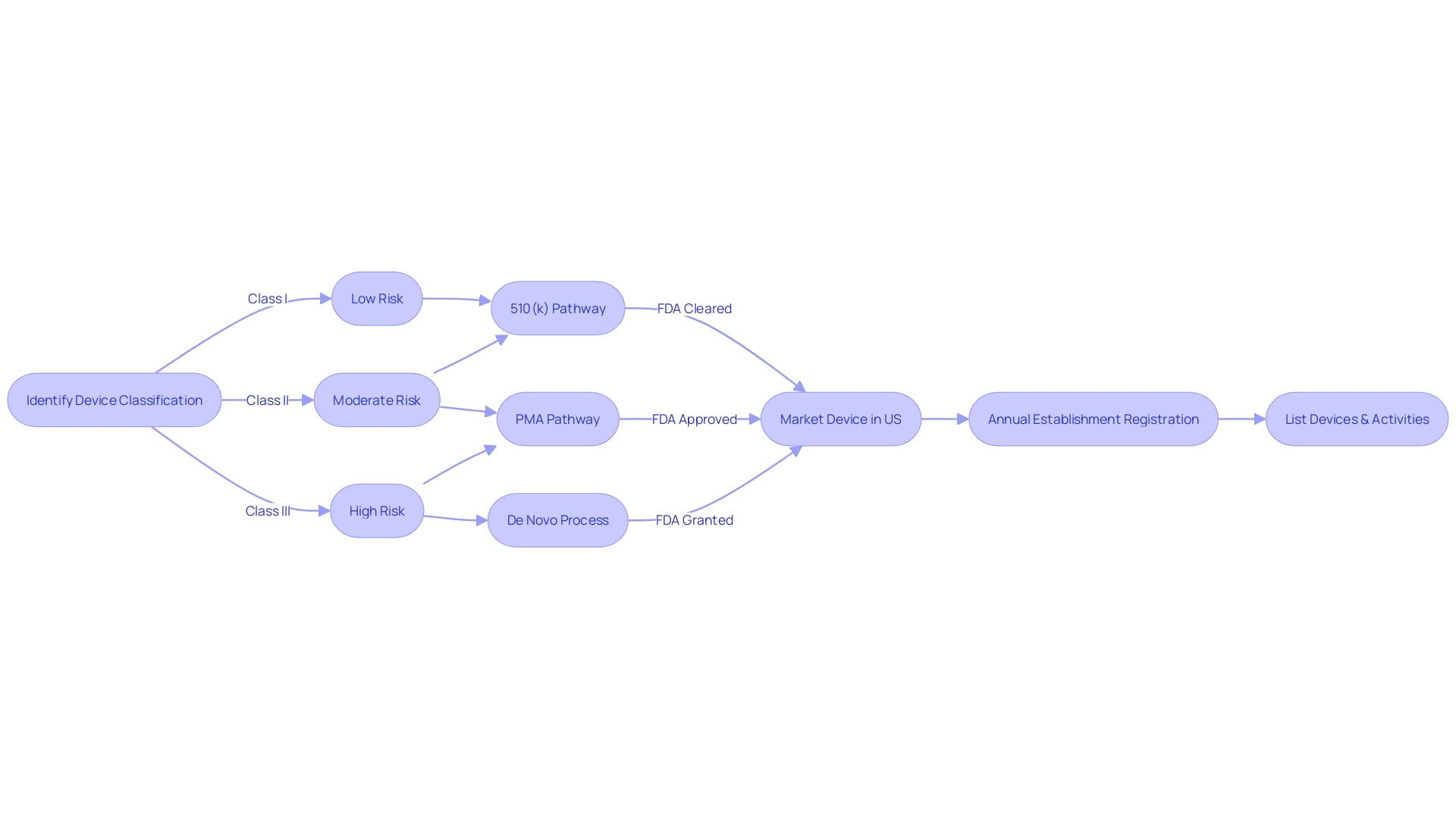
Navigating the FDA's regulatory landscape is essential for medical devices to ensure they meet the agency's stringent standards for safety and effectiveness before they can be marketed in the United States. The FDA classifies medical devices into three categories based on the risk they pose to patients, with class I representing the lowest risk and class III the highest. Devices in class I and II typically undergo the 510(k) clearance process, which involves demonstrating that the new device is substantially equivalent to a predicate device that has already been legally marketed.
Class III devices, which are often crucial to sustaining life, such as heart valves and implantable pacemakers, undergo a more rigorous Pre-Market Approval (PMA) process. This process requires a demonstration of safety and effectiveness, often including data from clinical trials.
In recent years, there has been a significant focus on improving the efficiency of these regulatory pathways to more rapidly address critical health issues. For example, the advent of devices that automate insulin delivery represents a breakthrough for individuals with diabetes, a condition affecting over 11% of Americans. This innovation exemplifies FDA's commitment to fostering medical advancements while ensuring public safety.
Additionally, the FDA's recent final rule on direct-to-consumer prescription drug advertisements emphasizes the importance of presenting information in a clear and accessible manner, ensuring that potential risks are communicated effectively to consumers.
It's crucial for industry professionals to understand the distinctions between being FDA 'Registered', 'Cleared', 'Approved', or 'Granted' status, as each term indicates a different level of FDA review and authorization. The De Novo process, for example, offers a pathway for novel devices that are not equivalent to an existing device to be 'Granted' marketing authorization. Understanding these differences can streamline the regulatory process and help bring innovative medical devices to market more efficiently.
FDA Approval
The FDA is a cornerstone in the safeguarding of public health, responsible for the rigorous evaluation of medical devices for safety and effectiveness, especially for those that carry high risks, such as life-sustaining equipment or implants. One exemplary case illustrating the FDA's commitment to this responsibility is the scrutiny of the Impella Connect System. This system, which includes both hardware and software components, is utilized for temporary ventricular support in critical care settings.
It was subjected to a stringent review process that examined its ability to monitor individual Automated Impella Controller (AIC) pumps, provide on-demand case information, and issue critical, time-sensitive alarms that can signal life-threatening conditions.
The FDA's device classification system categorizes devices into three classes based on risk, with class three devices like the Impella Connect System requiring a Pre-Market Approval (PMA) due to their significant role in life support. These devices must demonstrate their safety and efficacy through comprehensive clinical trial data and a review of their manufacturing processes and labeling before they can receive FDA approval.
Moreover, the FDA's oversight extends to the broader healthcare technology industry, as demonstrated by companies like Medtronic, which emphasizes its commitment to transforming lives through inventive medical technologies, such as cardiac devices and insulin pumps.
Regulatory professionals often encounter terms like 'Registered,' 'Cleared,' 'Approved,' and 'Granted', each signifying different levels of FDA evaluation and endorsement. The distinctions between these terms are essential to understand, as they reflect the regulatory pathway and the degree of scrutiny a device has undergone. For instance, a 510(k) clearance involves comparing a new device to an existing one, while PMA is a more exhaustive process reserved for class three devices that have a significant impact on patient health outcomes.
The urgency for accelerated approval pathways has been highlighted by the COVID-19 pandemic, prompting regulatory agencies and the healthcare industry to enhance collaboration and streamline processes for devices that meet critical healthcare needs, especially in emerging areas like digital health.
The FDA's role in maintaining the safety and security of medical devices is a dynamic and critical part of healthcare, ensuring that every device reaching the market has been rigorously evaluated to meet the highest standards of safety and effectiveness for patient care.
FDA Clearance
FDA clearance specifically refers to the process where the FDA reviews Class I and Class II medical devices, which are generally considered lower-risk. This evaluation is conducted through the Premarket Notification pathway, also known as the 510(k) process. During this process, the FDA assesses the device's intended use, design specifications, safety, effectiveness, and performance data.
A key aspect of the clearance is demonstrating that the new device is 'substantially equivalent' to a predicate device that is already legally marketed in the United States. The clearance is granted when the FDA is satisfied that the new device is at least as safe and effective as the existing device. It is noteworthy that not all devices cleared through the 510(k) process require clinical trials, which was a point of examination in the 2018 documentary 'The Bleeding Edge'.
The documentary highlighted instances where the fast-tracking of certain devices under this process led to patient harm. This underscores the importance of thorough review and vigilance in the clearance process to ensure patient safety and device efficacy.
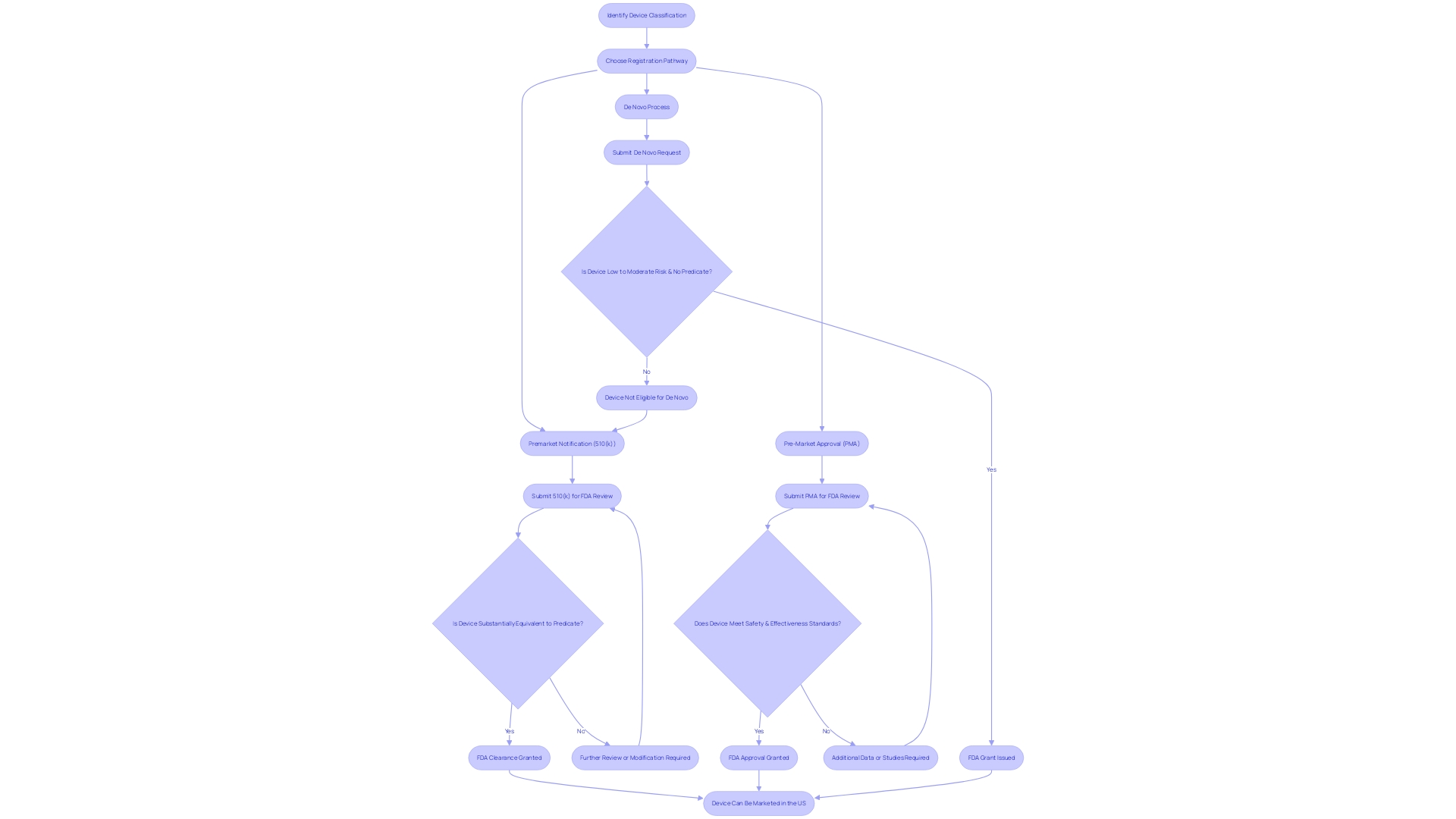
Key Differences Between FDA Approval and Clearance
The Food and Drug Administration (FDA) plays a pivotal role in safeguarding public health by ensuring the safety, effectiveness, and security of medical devices. Understanding the differences between FDA approval, clearance, and compliance is essential for medical device manufacturers and healthcare professionals. The FDA categorizes medical devices into three classes based on risk, with Class I representing low-risk devices and Class III including those that sustain or support life, such as implantable devices.
Class III devices, due to their high-risk nature, necessitate FDA approval, which involves an exhaustive review of clinical trial data to demonstrate safety and efficacy. For example, the Automated Impella Controller (AIC), a device for temporary ventricular support, had to undergo rigorous evaluation before receiving approval. Approval ensures that a device's health benefits outweigh any potential risks.
In contrast, Class I and II devices may be eligible for FDA clearance through the 510(k) process, which requires demonstrating that the device is substantially equivalent to a previously cleared predicate device, thereby reducing the need for extensive clinical trials. This was the case with the Impella Connect System, which allows remote monitoring of AIC pumps and has software functions that required premarket authorization due to their critical role in patient care.
The FDA also ensures that medical information, such as notifications of alarms from devices, is presented in a clear, conspicuous, and neutral manner to healthcare providers, as seen with the Impella Connect System's email notifications and color-coded alarm states.
The level of scrutiny and the pathway for registration, whether it's Premarket Notification (510(k)), Premarket Approval (PMA), or the De Novo process, is determined by the device's classification. The time and cost associated with these processes vary; the approval process is generally lengthier and more expensive due to the detailed clinical data required, while clearance can be a faster and less costly route to market.
To navigate these regulatory pathways successfully and ensure compliance, an understanding of the nuances between clearance, approval, and compliance is vital. Missteps in this process can lead to significant financial burdens, reputational damage, and delays in device availability. As such, staying abreast of FDA regulations and standards is a cornerstone in the development and marketing of medical devices.
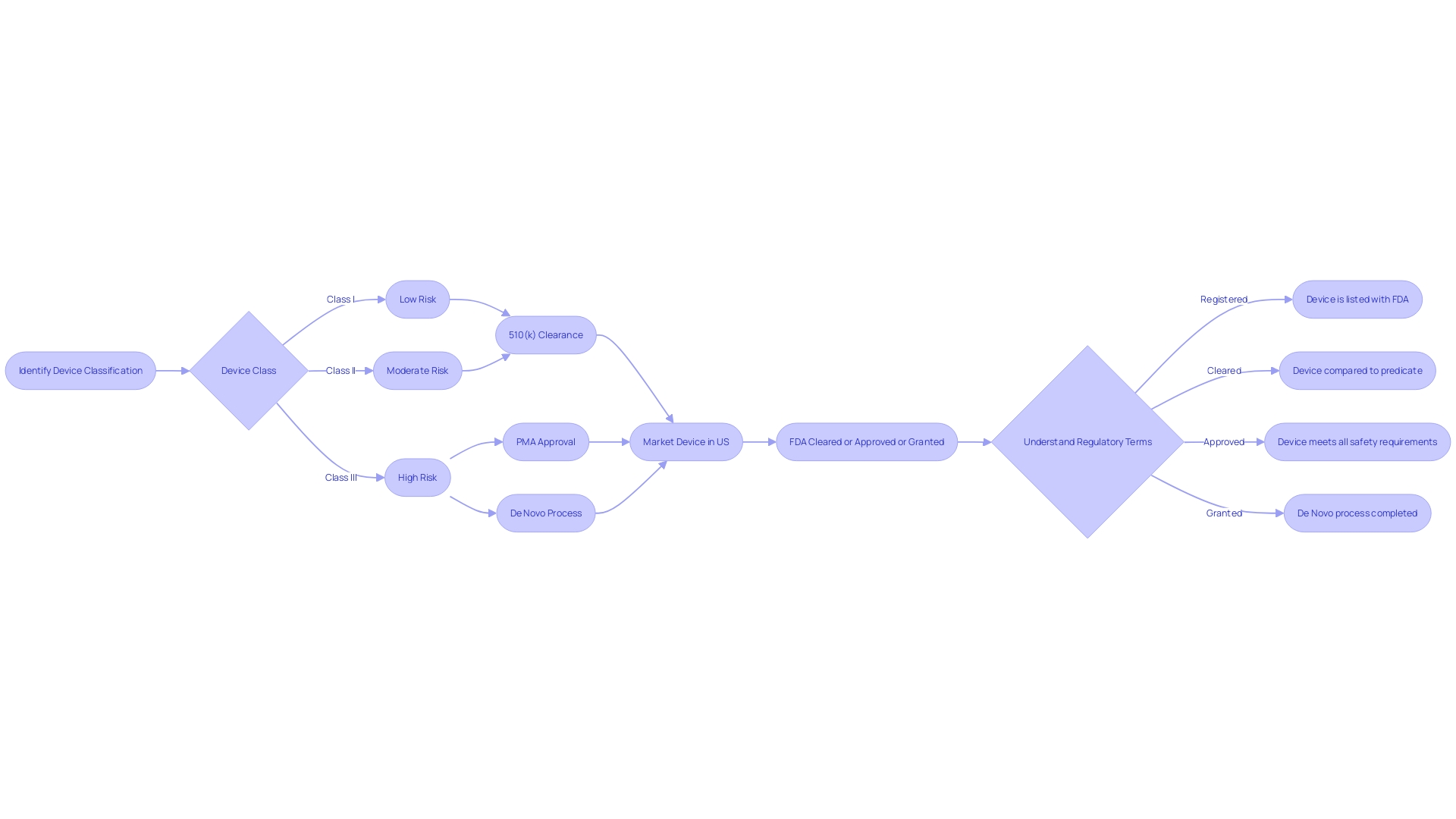
Implications for Consumers and Manufacturers
Navigating the regulatory environment of the FDA is essential for manufacturers and consumers alike, particularly when it comes to medical devices. The FDA classifies medical devices into three categories, reflecting the level of risk they pose to patients. Class I devices, deemed lowest risk, often require less regulatory control, while Class III devices, which include life-sustaining implants like pacemakers, are subject to the most stringent regulations due to their high risk.
Manufacturers must meticulously determine their device's classification to decide the appropriate registration pathway, which can be Premarket Notification (510(k)), Pre-Market Approval (PMA), or the De Novo process. With a 510(k) clearance, a device is compared to an existing device on the market, whereas PMA involves a more rigorous review, including clinical trials, to demonstrate safety and efficacy. The De Novo pathway is an option for novel low-to-moderate-risk devices that lack a comparable predecessor.
For consumers, FDA approval signifies that a device has met the highest standards of safety and effectiveness through thorough clinical trials. This provides greater confidence in the device's ability to perform as intended. Conversely, FDA clearance indicates that a device is substantially equivalent to another legally marketed device.
Understanding these regulatory nuances is not only critical for compliance and market entry but also impacts the timeline and cost associated with bringing a medical device to market. Incorrect or inadequate regulatory strategy can lead to delays, increased costs, and even the refusal of a device application. Therefore, both consumers and manufacturers must grasp these differences to make informed decisions and navigate the complexities of FDA regulations effectively.
Conclusion
In conclusion, understanding the FDA's regulatory pathways, such as approval and clearance, is crucial for industry professionals and healthcare providers. This article has highlighted the rigorous evaluation processes that medical devices undergo to ensure safety and effectiveness.
By navigating these pathways accurately, professionals can streamline the regulatory process and bring innovative medical devices to market more efficiently. The implications of FDA approval and clearance are significant for both manufacturers and consumers, providing confidence in a device's performance and ensuring patient safety.
Navigating the FDA's regulatory environment is essential for manufacturers and consumers alike. Understanding the differences between FDA approval, clearance, and compliance is vital for compliance, market entry, and informed decision-making.
In conclusion, a comprehensive understanding of the FDA's regulatory pathways is necessary to ensure the safety and effectiveness of medical devices. By navigating these pathways accurately, manufacturers can bring innovative devices to market efficiently, benefiting patient care.




