Introduction
IDE devices, or Investigational Device Exemption devices, are instrumental in medical research, serving as pivotal tools for evaluating the safety and effectiveness of new medical devices. These devices play a crucial role in advancing medical science, providing vital information on device performance and potential patient benefits. The development of IDE devices requires a comprehensive, multi-disciplinary approach, considering factors such as optical design, electrical and mechanical considerations, and software integration.
This article explores the significance of IDE devices in medical research, the IDE application process, the components of an IDE application, the types of devices requiring IDE approval, the responsibilities of investigators and sponsors, the role of Institutional Review Boards (IRBs), and the importance of Good Clinical Practices (GCP). Understanding IDE devices and their role in medical research is key to navigating the complex landscape of medical device development and ensuring the highest standards of safety and efficacy.
What are IDE Devices?
'Investigational Exemption (IDE) instruments are at the forefront of medical research, serving as pivotal tools in the evaluation of safety and effectiveness for new medical devices.'. These instruments are referred to as 'investigational' as they have yet to receive regulatory approval for public availability. Their role extends beyond mere data collection; they are integral in the progression of medical science, contributing vital information on equipment performance and potential patient benefits.
Throughout the creation of these gadgets, a cross-disciplinary approach is crucial. The complexities of optical design in IDE devices, for example, necessitate a systems-level viewpoint. It's not just about the optics; electrical and mechanical considerations are equally crucial, ensuring that the imaging output is seamlessly integrated with software systems and the apparatus is user-friendly for patient interaction.
The optical systems within IDE mechanisms must be carefully tailored to their intended environment and application. This includes establishing appropriate cleanliness standards during development, which may be less stringent initially but will need to comply with final product specifications. Furthermore, the choice of light sources is a vital design decision, with factors like wavelength, power, and illumination type playing a crucial role in both functionality and safety.
The process from idea to market for medical equipment is filled with complexities. Companies with a history of successful product launches demonstrate an adeptness in navigating this process, which is particularly relevant in the current era of digital health. These companies often have the advantage in integrating software with hardware, a crucial competency in today's medical equipment market.
Recent news underscores the importance of IDE instruments in clinical trials, such as the evaluation of MRI scan quality and advancements in thrombectomy procedures. These examples highlight the significance of IDE tools in not just influencing treatment practices but also in the broader approval process for new medical technologies.
As the medical technology sector evolves, developers play a crucial role. The global developer community is expanding, with the number of professional developers reaching approximately 13.4 million by 2023. This growth reflects the increasing democratization of development and the diversity of roles within the tech industry, from conventional programming to design and analysis, all of which contribute to the innovation in medical equipment development.
In summary, IDE mechanisms are crucial to the progression of medical research, offering a basis for evidence-based mechanism performance and patient benefits. The advancement of these technologies requires a thorough, interdisciplinary method, taking into account the incorporation of various systems and components to satisfy strict regulatory criteria. The successful navigation from concept to market entry is indicative of a company's expertise and capability to innovate in the dynamic field of medical equipment development.
Understanding the IDE Application Process
The path to integrating Investigational Device Exemptions (IDE) into clinical studies is a meticulous procedure that starts with an application to a governing body, such as the FDA in the U.S. This application is a vital document, outlining the equipment in question, the protocols of the intended examination, and the credentials of the investigative team. The FDA's Center for Drug Evaluation and Research (CDER) plays a crucial role, scrutinizing the application to confirm the scientific validity of the research, the safety of the device, and the balance of potential benefits against associated risks. The process aims to foster innovation in healthcare while ensuring patient safety and maintaining rigorous standards.
In the spirit of advancing medical knowledge and enhancing patient outcomes, the application process is supported by a strong framework of investigation and collaboration. For example, the work of Professor Jeffrey Ojemann at the University of Washington School of Medicine and Professor Steven Cramer from the University of California Los Angeles, funded by NIH, exemplifies the type of pioneering studies that can emerge when rigorous safety data and therapeutic strategies are developed through the IDE framework.
Moreover, the development and sharing of investigation tools, including the use of IDE devices, are increasingly reliant on open and collaborative environments, as indicated by initiatives like arXivLabs, which espouses values such as openness and community. These values are echoed by IT and data science experts at companies like Scieneers, who emphasize the importance of features such as reliable refactoring capabilities in software tools like PyCharm, which are instrumental in developing and deploying data solutions integral to clinical investigations.
As scholars navigate the application process, they are also encouraged to consider the broader implications of their work, including the dissemination and promotion of their studies. This involves not only adhering to best practice standards for software sharing but also actively engaging with communities that support these practices. The objective is to guarantee that the software for investigation is open, FAIR (Findable, Accessible, Interoperable, and Reusable), of high quality, and secure, thus maximizing the potential impact on biomedical, behavioral, and social sciences inquiry.
Ultimately, the IDE application process is not just a regulatory hurdle; it is a gateway to innovation and collaboration in clinical investigation, aimed at delivering new treatment options to patients and contributing to the advancement of global health care.
Components of an IDE Application
An Integrated Development Environment (IDE) is essential in the realm of clinical investigations, offering a sophisticated platform to handle various aspects of a study. An IDE typically encompasses:
-
This encompasses a thorough description of the IDE apparatus's design, materials, functionalities, and intended applications. It's a critical component as it aids regulatory authorities and investigative teams comprehend the device's capabilities and limitations.
-
Investigator Information: This section provides information about the qualifications and knowledge of the team involved. A proficient team, like the data experts from Scieneers, is essential for the successful implementation of a research project. Their expertise in data analysis, model development, and deployment of data pipelines is an illustration of the qualifications needed for a high-caliber team.
-
Study Protocol: It outlines the study's aims, methods, and procedures for data collection. A strong protocol is similar to a well-defined roadmap that guides the investigation from inception to conclusion, ensuring the collection of valid and reliable information.
-
Risk Assessment: A comprehensive evaluation of the potential risks and benefits of the IDE instrument is carried out. This reflects the feelings of industry professionals who stress the significance of comprehending the consequences of equipment usage in research environments.
-
Informed Consent Process: Describes the process of enlightening participants about the study and their rights. This is akin to the personalized care plans provided by companies like Proxie, which ensure individuals are fully informed and supported throughout their care.
-
Data Analysis Plan: This outlines strategies for evaluating collected data to ascertain the device's safety and efficacy. Similar to the analytical techniques utilized by Scieneers, a careful data analysis plan is essential for obtaining actionable insights from data.
Integrating these components into an IDE application enables a comprehensive approach to clinical investigation, similar to the way Scieneers or Proxie combine various services to improve value and care quality. Furthermore, with the growing intricacy of clinical research, such as those involving mechanical thrombectomy and advanced imaging, the importance of an IDE in streamlining the development process and adhering to regulatory documentation is more crucial than ever. This comprehensive framework ensures that every phase of the research is meticulously planned and executed, reflecting a commitment to excellence and patient safety.
Types of Devices Requiring IDE Approval
The classification of medical equipment into significant risk (SR) and non-significant risk (NSR) is a crucial stage in determining the regulatory pathway for their utilization in clinical studies. SR apparatus, such as the HeartMate 3 Left Ventricular Assist System, which provides crucial cardiac support, require Investigational Device Exemption (IDE) approval due to the potential risks they pose to participant health and safety. This strict requirement ensures that such equipment undergo thorough examination before being utilized in research environments.
Conversely, NSR instruments, which are considered to pose minimal risk to participants, do not require IDE approval. This category includes certain non-invasive diagnostic tools and basic monitoring equipment. However, it is crucial for all medical equipment, regardless of risk classification, to comply with relevant regulations and guidelines to guarantee participant safety and study integrity.
Comprehending the difference between SR and NSR gadgets is only the start. The FDA categorizes medical instruments into three levels based on patient risk factors, and each classification requires a distinct registration pathway, such as Premarket Notification (510(k)), Premarket Approval (PMA), or the De Novo process. For example, Software as a Medical Device (SaMD) that meets the criteria of a Class III product, such as the Wave Desktop, must adhere to strict standards, including software validation under 21 CFR 820.30(g), to guarantee conformity with FDA regulations.
The process of classifying and subsequently selecting the appropriate regulatory pathway is essential for the legal marketing of a product in the United States. Regulatory professionals must navigate the nuances between terms like Registered, Cleared, Approved, and Granted, which have significant implications for the marketing and use of medical products.
Moreover, the incorporation of consensus standards and their recognition by the FDA plays a vital role in the regulatory landscape, as outlined in the Federal Register and CDRH Learn resources. These standards provide a framework for manufacturers to demonstrate compliance and facilitate the approval process.
In the ever-evolving realm of medical technology, keeping up with the most recent advancements and regulatory modifications is essential. For example, recent discussions highlight the potential benefits of flow reversal during mechanical thrombectomy, despite the lack of randomized data. Such conversations highlight the significance of IDE approval in providing clarity to companies about the expectations and benchmarks for product approval.
The changing characteristics of medical equipment technology and regulation requires ongoing monitoring and adjustment by clinical investigation professionals to guarantee the utmost levels of patient safety and trial effectiveness.
Investigator and Sponsor Responsibilities
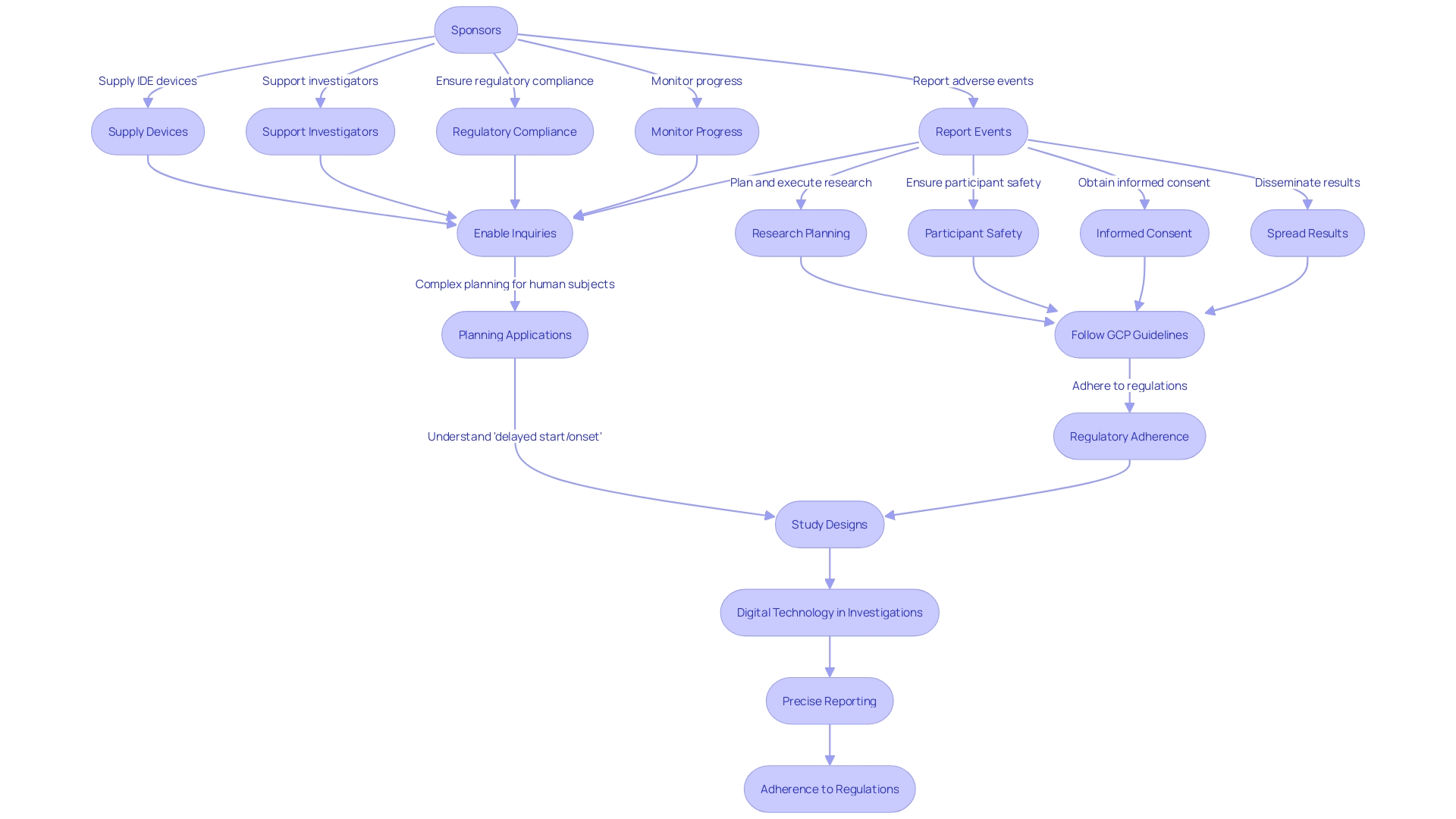
Integrated Device Electronics (IDE) devices are crucial for research investigations, where both investigators and sponsors play essential roles in guaranteeing the research's integrity and adherence to ethical standards.
Investigators have the obligation to plan and execute the research, which involves ensuring the safety of participants, obtaining informed consent, and overseeing data collection and analysis. They are also responsible for spreading the research results. To maintain the high standards of research, investigators must follow Good Clinical Practice (GCP) guidelines and meet regulatory requirements. For instance, the NIH peer review system evaluates applications for scientific and technical merit, with the R61/R33 phased innovation grant underlining the emphasis on novel scientific ideas without the necessity for preliminary data or extensive background material, provided there is a solid justification for the proposed work.
On the flip side, sponsors, who initiate and often finance the research, are responsible for supplying the IDE device and supporting the investigators. They must ensure that the research complies with regulatory mandates, monitor its progress, and report any adverse events or outcomes to the authorities. For instance, a recent investigation conducted by Jeffrey Ojemann and Steven Cramer, supported by the NIH, aims to create innovative therapeutic rehabilitation approaches for stroke patients, emphasizing the sponsors' contribution to enabling groundbreaking inquiries.
Additionally, the emergence of digital technology in investigations, such as wearable devices and smartphones, has brought forth fresh perspectives to data gathering and biomarker advancement, particularly in mental health studies. This emphasizes the requirement for precise reporting and adherence to regulations by both investigators and sponsors for the accomplishment of a research.
Scientists must also be mindful of the intricacies of planning applications that involve human subjects investigation, such as comprehending the distinctions between 'delayed start' and 'delayed onset'. A postponed beginning implies that the investigation on human subjects will commence later in the funding period with complete details incorporated in the application, while a delayed initiation indicates that the inquiry cannot be fully determined at the time of application and will necessitate preliminary findings to conclude the portion involving human subjects at a later stage.
The importance of these roles is further highlighted by the FDA's guidelines on the general responsibilities of investigators and the operation of data monitoring committees, ensuring the protection of human subjects and the integrity of the data collected during clinical trials. As the medical community continues to progress with new technologies and methodologies, the obligations of investigators and sponsors in research investigations remain essential to the progression of medical knowledge and the development of new treatments.
The Role of Institutional Review Boards (IRBs)
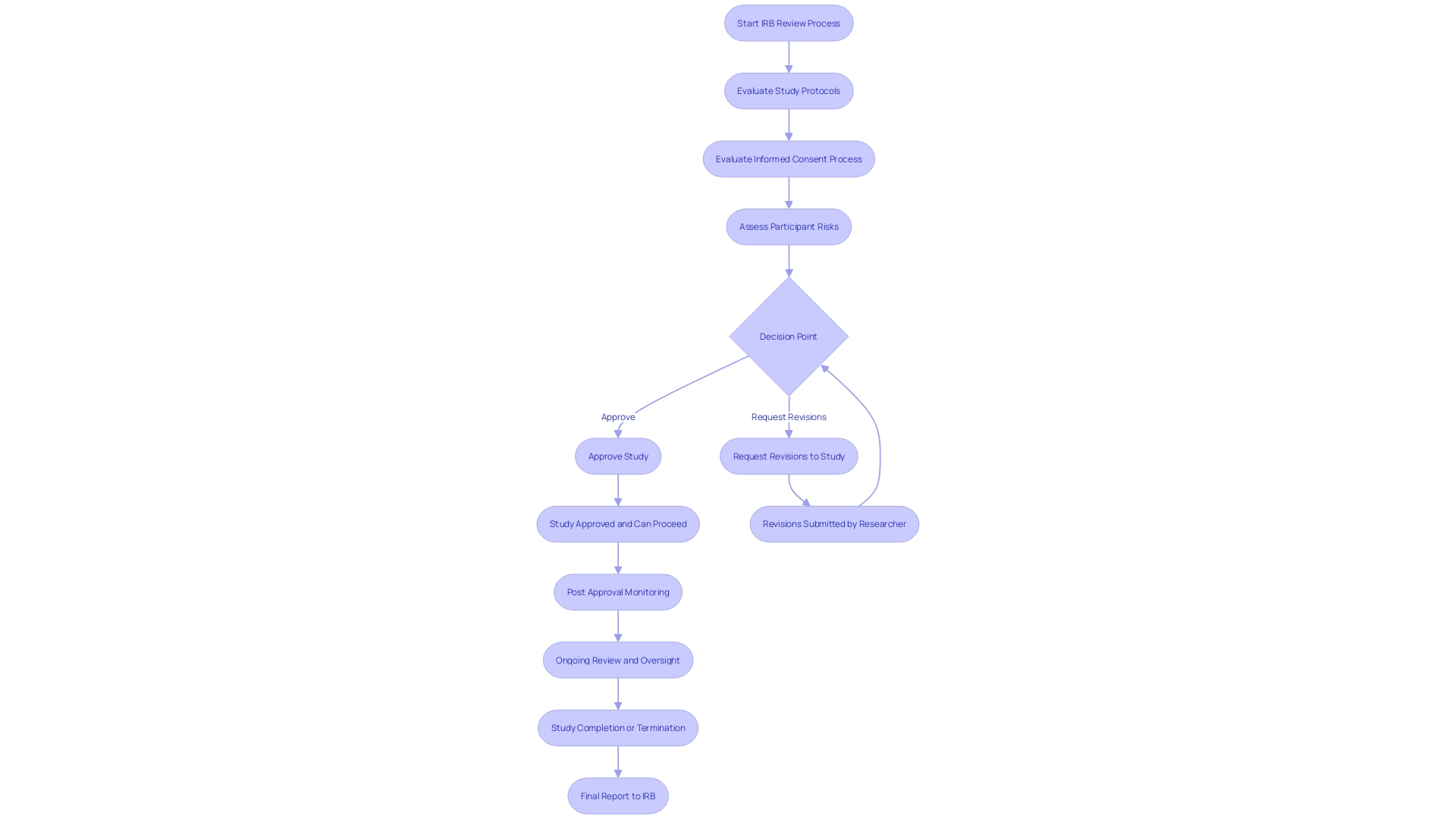
Institutional Review Boards (IRBs) are crucial in protecting ethical standards in research involving Investigational Device Exemptions (IDEs). Comprised of medical experts, researchers, and community advocates, these independent committees meticulously scrutinize study protocols, the informed consent process, and participant risk assessments. Their thorough examination ensures that studies adhere to ethical guidelines, upholds participant rights, and actively seeks to minimize potential harm.
IRBs came into the spotlight following the National Research Act of 1974, responding to historical ethical breaches such as the Tuskegee Syphilis Study. The Act recognized the need for increased protective measures, emphasizing the crucial role IRBs play in the progress of medical science. Their duties involve a comprehensive proposal evaluation, which may require requesting revisions—a procedure that can significantly prolong the timeline for investigation initiation.
Moreover, the importance of IRBs in the context of clinical trials cannot be overstated. With the majority of experimental treatments failing to provide direct benefits, IRBs justify the risks and burdens on volunteers through the potential for broader scientific advancements. The evaluation and endorsement of studies by IRBs is vital for upholding public trust and guaranteeing that volunteer contributions to medical science are ethically sound and valuable.
Good Clinical Practices (GCP) for IDE Studies
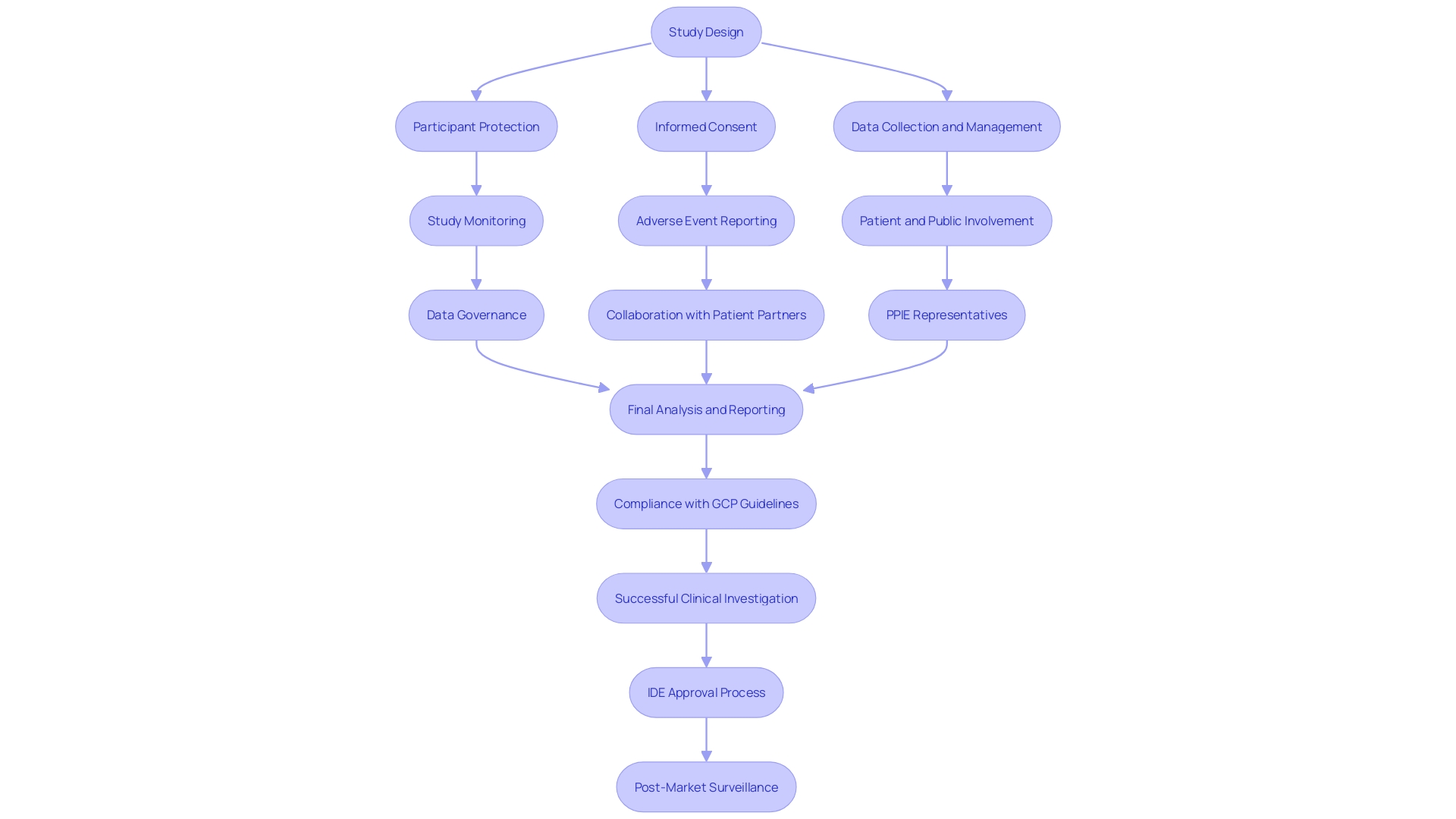
Following the Good Clinical Practice (GCP) guidelines is crucial in upholding the ethical and scientific standards of clinical investigations, particularly in trials involving Investigational Device Exemptions (IDE). These guidelines provide a comprehensive framework for the complete duration of an investigation, from its initial design through to its execution and eventual reporting. They are crucial in ensuring participant protection, informed consent, meticulous data collection and management, continuous study monitoring, and systematic adverse event reporting.
GCP guidelines have evolved to include patient and public involvement (PPIE) to enhance the relevance and patient-centeredness of studies. Involving patient partners—who could be individuals with personal experience, caregivers, or community representatives—during the design and conduct of studies has become an established approach, enhancing the significance of the findings. For example, the term ‘patient partner’ is used to signify patients or their informal caregivers actively engaged in the research process.
The recent draft update to the European GCP guidance, slated for implementation in late 2024, underscores the importance of data governance. This section emphasizes the need to prioritize participant safety, wellbeing, and data reliability, guiding researchers to carefully evaluate scientific objectives and associated risks.
MDCG 2024-3 offers comprehensive guidance on the information required for an investigation, including the description of the apparatus, type of inquiry, and the reasoning behind it. It also requires outlining the roles, responsibilities, and qualifications of all investigators, ensuring the investigational device's intended purpose is clear, and providing a thorough analysis of benefits and risks.
The integration of precision medicine terms in titles and abstracts signals the study's relevance to this field, and the inclusion of race and ethnicity, while controversial, remains vital due to their recognition by certain health authorities in the precision medicine context. The need for collaboration with PPIE representatives in addressing the potential impact of studies on target populations is increasingly acknowledged.
Modern GCP guidelines demonstrate a comprehension that embracing diversity in patient experiences and acknowledging the importance of patient engagement are crucial for the progress of clinical studies. This alignment with patient needs and the emphasis on data integrity serve as cornerstones for the continued evolution of clinical research methodologies.
Pre-IDE Process and Early Feasibility Studies
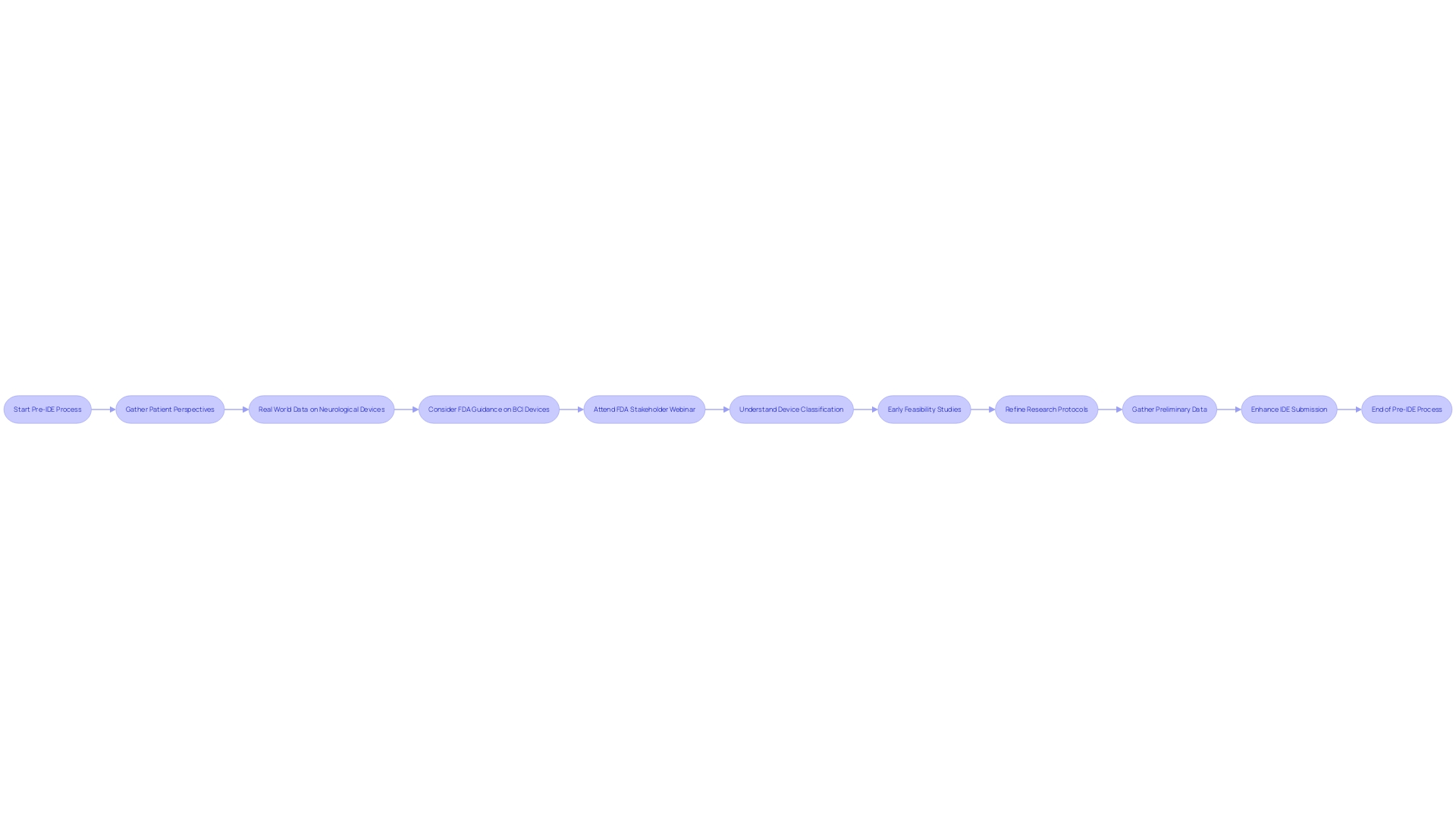
Participating in a pre-Investigational Device Exemption (IDE) process enables researchers to gather preliminary data essential for evaluating the feasibility of their investigation. This involves early feasibility studies—limited scope trials aimed at evaluating an IDE device's safety and efficacy. These initial phase investigations serve as a testing ground, allowing researchers to identify potential obstacles, refine research protocols, and gather foundational evidence to enhance the IDE submission.
The advantages of early feasibility investigations are backed by recent advancements in technology, such as the incorporation of AI in various applications, which emphasize the significance of adaptability and foresight in clinical research. The development of smaller, yet effective, devices like the Anaconda, which received IDE approval, illustrates the importance of innovation in medical device design. This aligns with the industry-wide recognition that successful outcomes often reside within the realm of strategic management and detailed planning.
To propose a robust clinical trial, researchers must present a hypothesis-driven approach, detailing the trial stages and establishing contingency plans. Previous research must validate the trial's necessity, its potential impact, and a comprehensive conceptualization that predicts the likelihood of success. Furthermore, the trial must adhere to stringent ethical standards and regulatory practices, such as Good Clinical Practices (GCP), to ensure the highest level of scientific integrity and patient safety.
These preparatory steps not only align with FDA’s Center for Drug Evaluation and Research (CDER) guidelines for clarity in study design but also reflect a broader industry trend towards innovative, scientifically rigorous medical products that address unmet medical needs and enhance patient care.
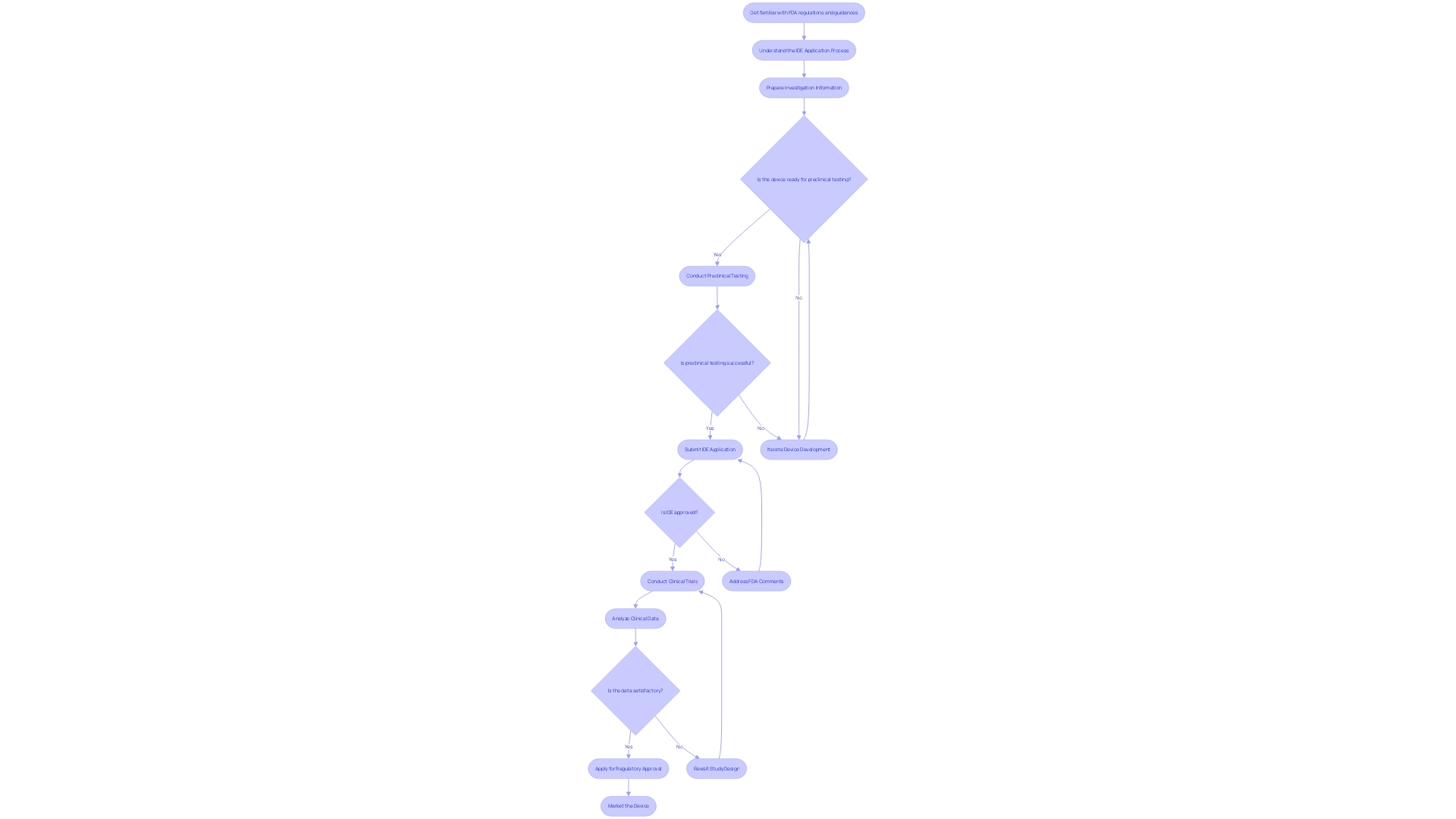
IDE Development Process for Sponsor-Investigators
As pioneers in the realm of clinical research, sponsor-investigators play a dual role in advancing medical technology through a complex and iterative process. This begins with the collaborative effort in instrument development, where sponsor-investigators unite with a diverse team of engineers, scientists, and medical experts to create an Investigational Device Exemption (IDE) tool. The aim is to guarantee the safety and effectiveness of the equipment from the beginning. After this, the apparatus must demonstrate its worth through rigorous preclinical testing. Utilizing both laboratory settings and animal models, this phase is critical for assessing performance, safety, and identifying potential risks.
The journey continues with early feasibility research, where initial safety and effectiveness data are collected. These studies are crucial for improving the apparatus and its intended purpose. Armed with preclinical and early feasibility data, sponsor-investigators navigate the regulatory landscape by submitting an IDE application to the governing authority, seeking approval to move forward with human trials.
After receiving the green light, clinical trials are conducted, expanding the evaluation of the IDE instrument's safety and effectiveness to a broader human population. This is a decisive stage where real-world data is gathered, which can lead to unexpected yet life-saving discoveries, as evidenced by participants like Barbara, who, through her involvement in a trial, uncovered a serious, symptomless heart condition.
Finally, if the clinical trials yield positive results, sponsor-investigators reach the regulatory approval stage. Here, the objective is to acquire the required approval to commercially market and sell the innovative instrument, enabling the advantages of the investigation to reach the public. This process is not linear but rather an iterative dialogue between development and testing, echoing the sentiments of academic advisors who advocate for an incremental approach to investigation and writing.
In the backdrop of these stages is a continuous process of learning and adaptation, as real-world applications and the integration of data science, such as the work by Scieneers, provide valuable insights into the design and execution of clinical trials. Furthermore, the field of medical equipment advancement is constantly changing, as demonstrated by recent experiments that have emphasized the significance of flow reversal in mechanical thrombectomy and the potential of AI to enhance the quality of MRI images. These advancements signify the dynamic nature of clinical research and the importance of iterative development and testing in creating impactful medical devices.
Conclusion
In conclusion, IDE devices are crucial for advancing medical research by evaluating the safety and effectiveness of new medical devices. The development of IDE devices requires a comprehensive, multi-disciplinary approach, considering optical design, electrical and mechanical considerations, and software integration.
The IDE application process involves submitting a detailed application to a governing body, such as the FDA, to ensure scientific validity, device safety, and the balance of benefits and risks. Adhering to Good Clinical Practice (GCP) guidelines is pivotal for upholding ethical and scientific standards in clinical research.
Investigators and sponsors have distinct responsibilities in IDE studies, ensuring participant safety, obtaining informed consent, and complying with regulations. Institutional Review Boards (IRBs) play a critical role in upholding ethical guidelines and participant rights.
Understanding the categorization of devices into significant risk (SR) and non-significant risk (NSR) helps determine the regulatory pathway. Adherence to regulations and guidelines is crucial for participant safety and study integrity.
Engaging in a pre-IDE process and conducting early feasibility studies are vital for assessing the viability of a study and collecting preliminary data. These steps refine study protocols and strengthen the IDE submission.
Overall, IDE devices and their development process are instrumental in advancing medical research. Collaboration between investigators, sponsors, and regulatory bodies, along with adherence to regulations and guidelines, ensures the highest standards of safety and efficacy in IDE studies and the development of innovative medical devices.




