Introduction
The journey from conception to approval of new medical therapies is complex and multifaceted. An Investigational New Drug (IND) application represents a pivotal moment in this process, serving as a formal request to the FDA for permission to initiate human trials for a new drug or biological product. This step is not simply procedural but is a critical juncture at which the safety and efficacy of a drug are first scrutinized in the context of human subjects.
The IND application, steeped in forward-looking statements, is grounded in a robust collection of preclinical data. It encompasses not only the anticipation of a drug's therapeutic potential but also a comprehensive understanding of its possible risks. The FDA's Center for Drug Evaluation and Research (CDER) plays an instrumental role in this phase, providing drug developers with necessary guidance on study design and data requirements.
Their expertise in the science behind new drugs, testing protocols, and the diseases targeted by these drugs is crucial for a thorough assessment.
In this article, we will explore the components of an IND application, the preparation process, the submission process, and the FDA's review and feedback. We will also delve into a case study highlighting the challenges and successes in IND submission, as well as lessons learned and best practices for a successful application. So, let's dive into the world of IND submissions and understand the intricate process behind bringing safe and effective therapies to patients.
What is an IND?
The journey from conception to approval of new medical therapies is complex and multifaceted. An Investigational New Drug (IND) represents a crucial moment in this process, acting as a formal request to the FDA for permission to start human experiments for a new drug or biological product. This step is not simply procedural but is a critical juncture at which the safety and efficacy of a drug are first scrutinized in the context of human subjects.
The IND, filled with forward-looking statements, is based on a strong collection of preclinical data. It encompasses not only the anticipation of a drug's therapeutic potential but also a comprehensive understanding of its possible risks. The FDA's Center for Drug Evaluation and Research (CDER) plays an instrumental role in this phase, providing drug developers with necessary guidance on study design and data requirements. Their expertise in the science behind new drugs, testing protocols, and the diseases targeted by these drugs is crucial for a thorough assessment.
A study's 'Translational Index score,' inspired by financial health credit scores, provides an innovative metric for assessing translational needs within the drug development framework. It encapsulates the readiness of a product to move from preclinical stages to human testing, thus guiding teams to address specific development requirements effectively.
The development pathway for drugs is filled with challenges, including regulatory submissions, initiation and completion of experiments, and the recruitment of subjects—all while managing the high costs associated with bringing a drug to market, which can range from $340 million to $2.8 billion. Drug developers must navigate this landscape with precision and care, understanding that each step, from IND submission to final approval, is integral to the ultimate goal of delivering safe and effective therapies to patients.
Components of an IND Application
An Investigational New Drug (IND) application is crucial in the research process, involving vital components that guarantee a comprehensive assessment of the investigational product and the intended study. These essential components include:
-
Investigator's Brochure: This document serves as a comprehensive repository of information about the investigational product. It encompasses the pharmacology, toxicology, and data from prior medical studies, enabling a clear comprehension of the product's safety and efficacy profiles.
-
Chemistry, Manufacturing, and Controls (CMC) Information: This section provides intricate details regarding the investigational product’s manufacturing process, the composition, stability, and protocols for quality control. This guarantees that the product maintains a consistent level of quality and effectiveness throughout the testing period.
-
Preclinical Data: The safety and potential efficacy of the investigational product are substantiated through results derived from preclinical animal studies. This data forms the foundation of the application, supporting the shift to human clinical experiments.
-
Clinical Trial Protocol: A meticulously detailed plan is laid out in this section, delineating the experiment's objectives, design, methodology, and the statistical considerations that will be used for analysis. This protocol is the blueprint for conducting the experiment, ensuring that it meets rigorous scientific standards.
-
Ethical considerations are of utmost importance in clinical research. These forms provide potential participants with comprehensive information regarding the study, including both potential risks and benefits, empowering them to make well-informed decisions about their participation.
The IND submission is supported by a structured approach that mirrors the rigor and clarity expected in business requirements specifications for IT systems. Just as a core system is broken down into subsystems and modules for clarity, the IND components are distinctly categorized to cover all aspects of the investigational product and trial. Involving relevant parties, like researchers in the medical field, during the IND preparation guarantees that the submission is both precise and representative of the shared knowledge.
Considering the latest updates from Intellia, the significance of a strong IND submission is emphasized by the statements about their experimental gene editing treatments. The organized approach to their IND submissions could be a contributing factor to their assurance in commencing worldwide Phase 3 studies and progressing their programs.
Furthermore, the IND process involves critical evaluation of the study's rationale, just as a foreign national seeking a B-1 visa for business purposes must justify their trip. In both scenarios, the importance of the objective is crucial—whether it's the effect of an experiment or the motive for journey.
Lastly, a quote that resonates with the IND process states, 'Effectiveness is entirely context dependent.' This is similar to the IND submission, where the circumstances of the experimental product, as determined by research study designs and regulatory criteria, influence its likelihood of achievement. Therefore, the IND must be meticulously developed to consider the particular context in which the investigational product is to be assessed.
Preparing for IND Submission
Ensuring a medication is secure and potentially efficient before it reaches experiments is a multi-layered process, involving an assortment of preclinical studies. This groundwork is not only a regulatory requirement but a vital step in safeguarding patient safety. Preclinical research is typically divided into in vitro (outside a living organism) and in vivo (within a living organism) studies. These studies are essential for gathering data on the investigational product's safety and efficacy. For instance, Neuralink categorizes its preclinical research into bench, pilot, R&D, and GLP studies. Each category serves a unique purpose, from proof-of-concept to testing that aligns with existing standards and establishes readiness for regulatory compliance.
When preparing an IND, it's crucial to possess a thorough study plan and a precise researcher's brochure in hand. Cardinal Health's partnership with a client to establish a regulatory starting material strategy exemplifies the meticulous planning required for a successful IND submission. This strategic approach is crucial for aligning preclinical research with regulatory expectations and ensuring that all necessary documentation, such as informed consent forms and Chemistry, Manufacturing, and Controls (CMC) information, is systematically compiled.
The significance of these preparatory stages is highlighted by the FDA Modernization Act 2.0, which defines nonclinical testing in broad terms that include a variety of test methods. The ultimate objective is to support a seamless shift into the testing phase, backed by strong and extensive data. As reflected in AbbVie's ongoing efforts to make a remarkable impact beyond medicine, recognized by industry accolades, the commitment to rigorous preclinical testing is a cornerstone of advancing medical research responsibly.
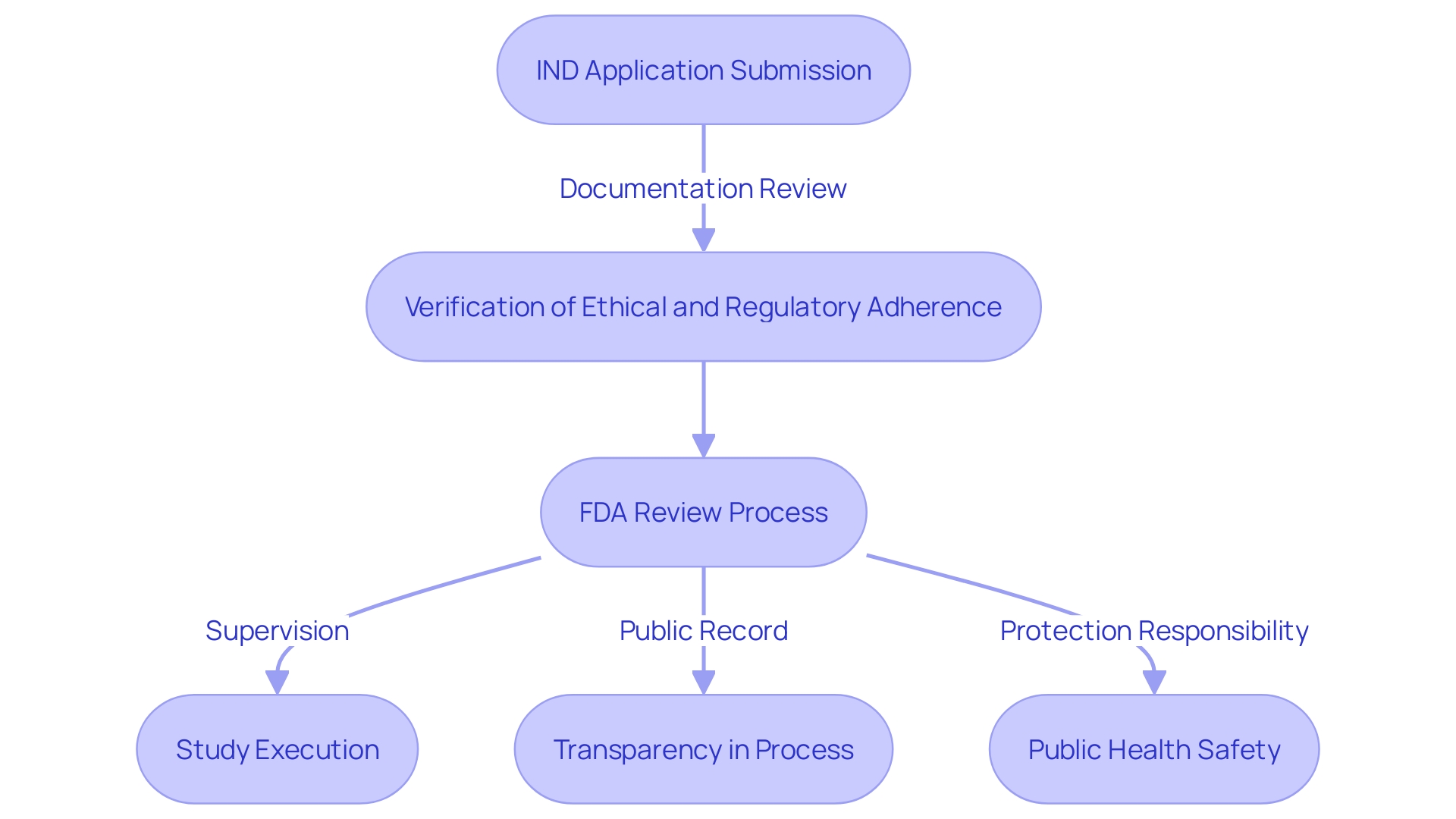
IND Submission Process
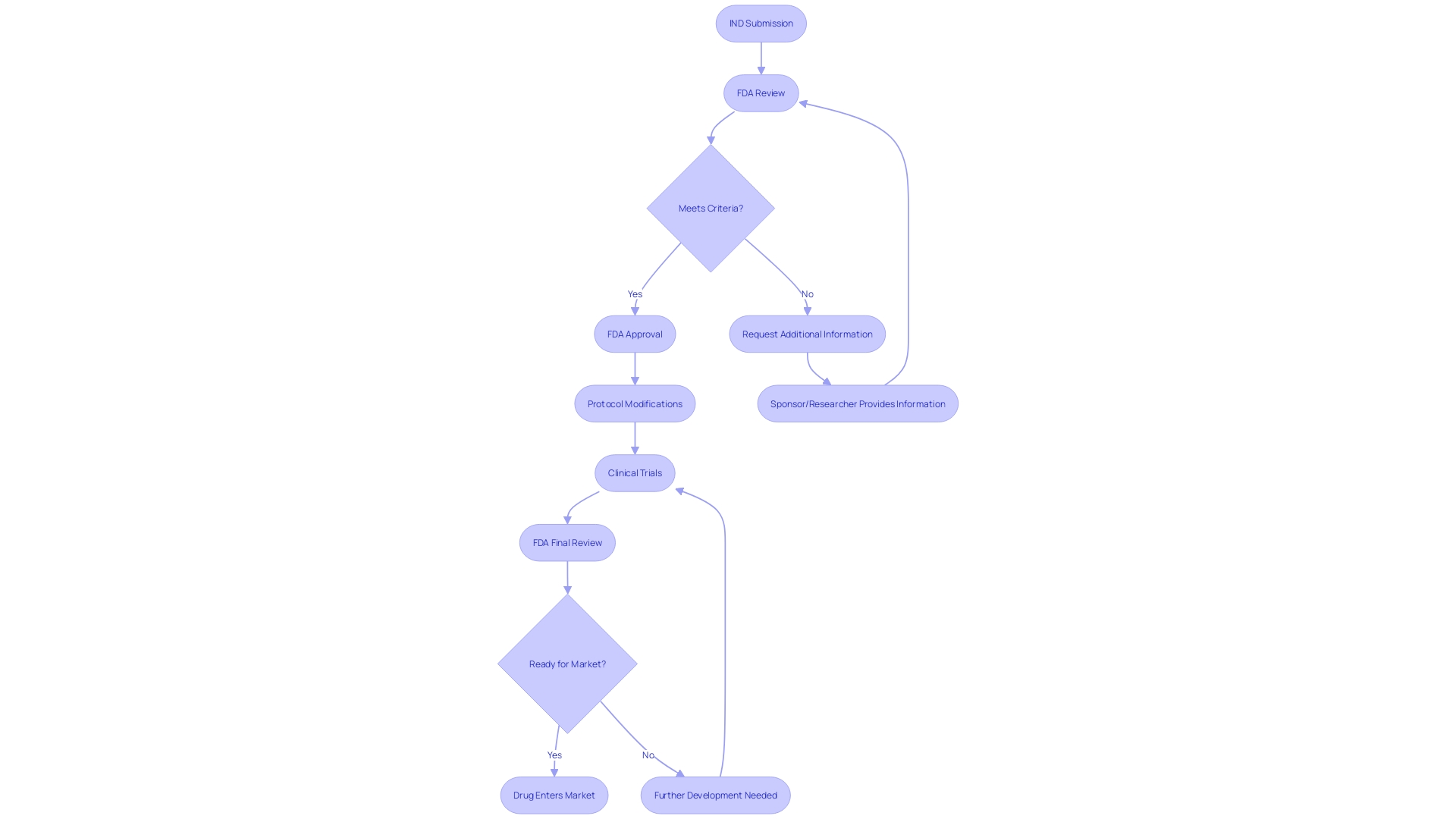
When a new Investigational New Drug (IND) application is submitted to the FDA, the agency meticulously reviews the documentation to confirm adherence to ethical and regulatory requirements. This process is crucial for protecting public health and ensuring that clinical experiments for new medical treatments are conducted responsibly. The FDA's review is designed to verify the integrity of the information provided and ascertain that the proposed experiment will not pose undue risk to participants.
The FDA's responsibility goes beyond the initial review of the submission, as they also supervise the execution of the study once authorized. It is essential for applicants to understand the gravity of this process and the importance of transparency. Any comments or documentation submitted electronically become part of the public record, underscoring the responsibility of applicants to omit confidential or sensitive information unless it is submitted following the FDA's guidelines for written/paper submissions.
Moreover, adherence to the FDA’s standards is evident in other areas, such as the regulatory requirements for direct-to-consumer prescription drug advertisements. The recent final rule by the FDA stipulates that the major statement in television and radio advertisements must be presented in a way that is clear, conspicuous, and neutral, using consumer-friendly language to ensure comprehension.
Ultimately, the thoroughness of the FDA’s review process is reflected in the agency's broader role in protecting public health by assuring the safety and efficacy of human and veterinary drugs, medical devices, and other products. This comprehensive approach is aimed at maintaining trust and safety in medical advancements and consumer products alike.
Post-Submission: FDA Review and Feedback
After receiving an Investigational New Drug (IND) submission, the FDA thoroughly examines the documentation to guarantee that the suggested clinical study fulfills all essential safety and effectiveness criteria. If the FDA needs additional information, they will notify the sponsor or researcher, who will then be responsible for providing the requested details or making any necessary modifications to the protocol. This iterative process is crucial for aligning with FDA's rigorous standards, which are in place to protect public health by confirming the safety, effectiveness, and security of human and veterinary drugs, vaccines, and other biological products, as well as medical devices. It's important for sponsors to follow FDA's guidance closely, including the submission of a clear and concise cover letter along with their scientific evaluation, to facilitate a smooth review process. The evaluation should address whether the new drug could present any safety concerns, such as allergenic or toxic properties. Ultimately, the goal is to resolve all potential issues before the product enters the commercial market, ensuring that any new therapeutic agents available to the public have been thoroughly vetted by the FDA.
Case Study: Challenges and Successes in IND Submission
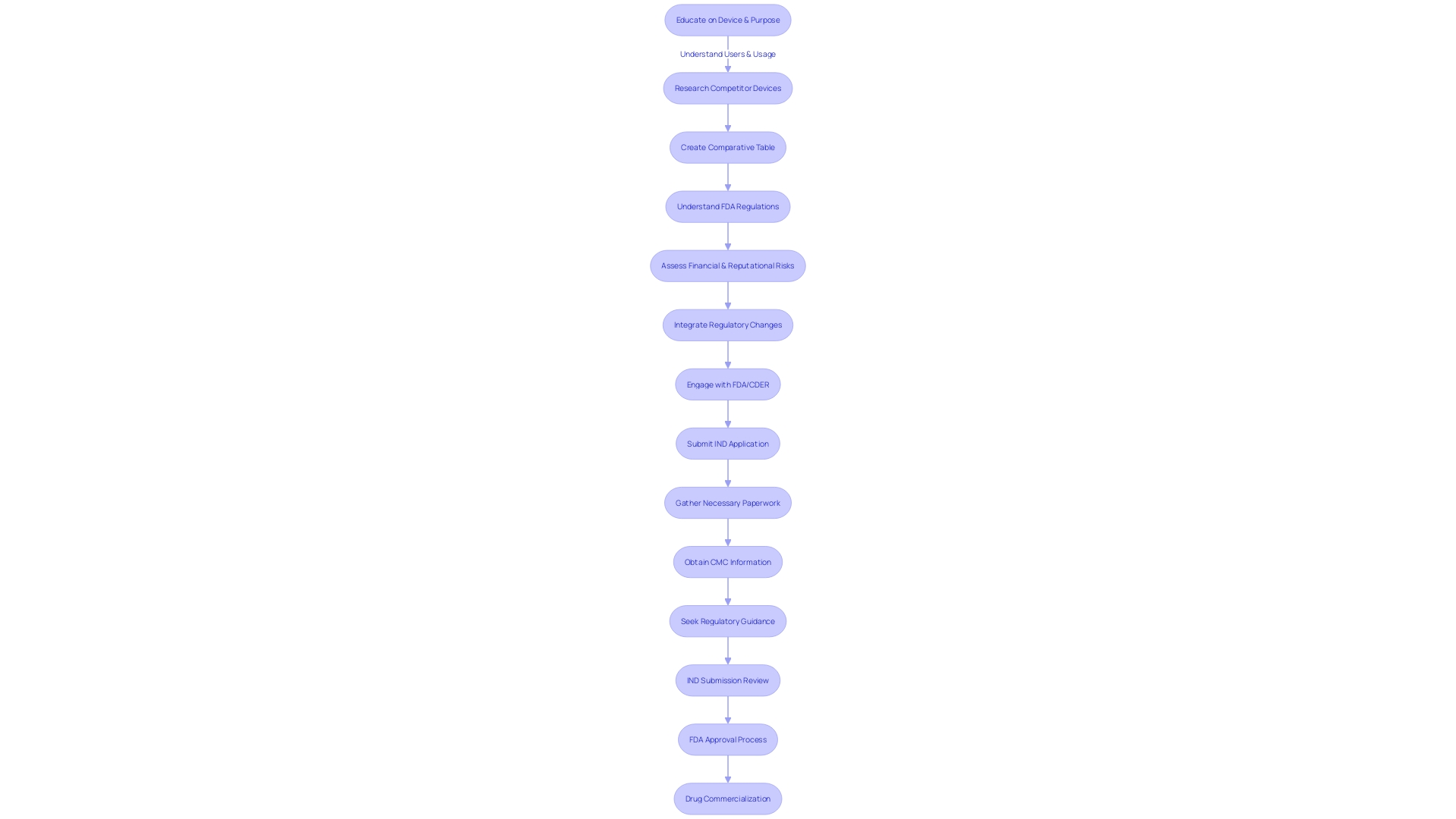
Understanding and complying with the intricate regulatory framework for investigational drug submissions can be a challenging undertaking, as evidenced by a recent encounter of a biotech company collaborating with Cardinal Health. Despite comprehensive preclinical research and a specified protocol, the company struggled with gathering all necessary paperwork and experienced delays in obtaining essential Chemistry, Manufacturing, and Controls (CMC) information from manufacturing partners. Nonetheless, they surmounted these hurdles with the aid of seasoned regulatory advisors, resulting in a successful Investigational New Drug (IND) submission.
The biotech industry's reliance on high-quality data is crucial, particularly in the computational realm where proprietary or defensible data modalities form a technological moat. This industry's efforts, as seen in the collaborative initiative between Benchling and the Allotrope Foundation, are focused on establishing data standards that bolster R&D efficiency. The initiative's open-source library for harmonizing instrument data underscores the industry's commitment to enhancing data interoperability and AI-readiness.
The importance of data integrity and the organization of the data team are pivotal. A company's infrastructure stack, whether built internally or sourced externally, and the rationale behind the selected tools play a significant role in determining the scalability and cost-effectiveness of its data stack. In this context, Dr. Lisa Hess's retrospective study on mantle cell lymphoma offers a glimpse into the potential of well-organized data in informing treatment patterns in areas with limited guidance.
As regulatory submissions become increasingly digitized, it's imperative to follow proper protocols when submitting comments or confidential information to public dockets. The Food and Drug Administration (FDA) provides clear instructions to prevent the public disclosure of sensitive data, which emphasizes the responsibility of individuals and organizations in maintaining confidentiality.
In the realm of technology assessment, it is essential to weigh the utility of a technology against its capabilities. Discussions on governing genome editing and dual-use technologies highlight the need for innovative governance approaches that are responsive to the rapid advances in life sciences. The ongoing dialogue aims to shape effective policies that address security considerations and ethical implications of genome editing advancements.
Lessons Learned and Best Practices for IND Submission
Navigating the intricacies of Investigational New Drug (IND) submissions requires strategic planning and attention to detail. Drawing from a well of collective experience and a recent case study, several core strategies have been identified to streamline the IND filing process.
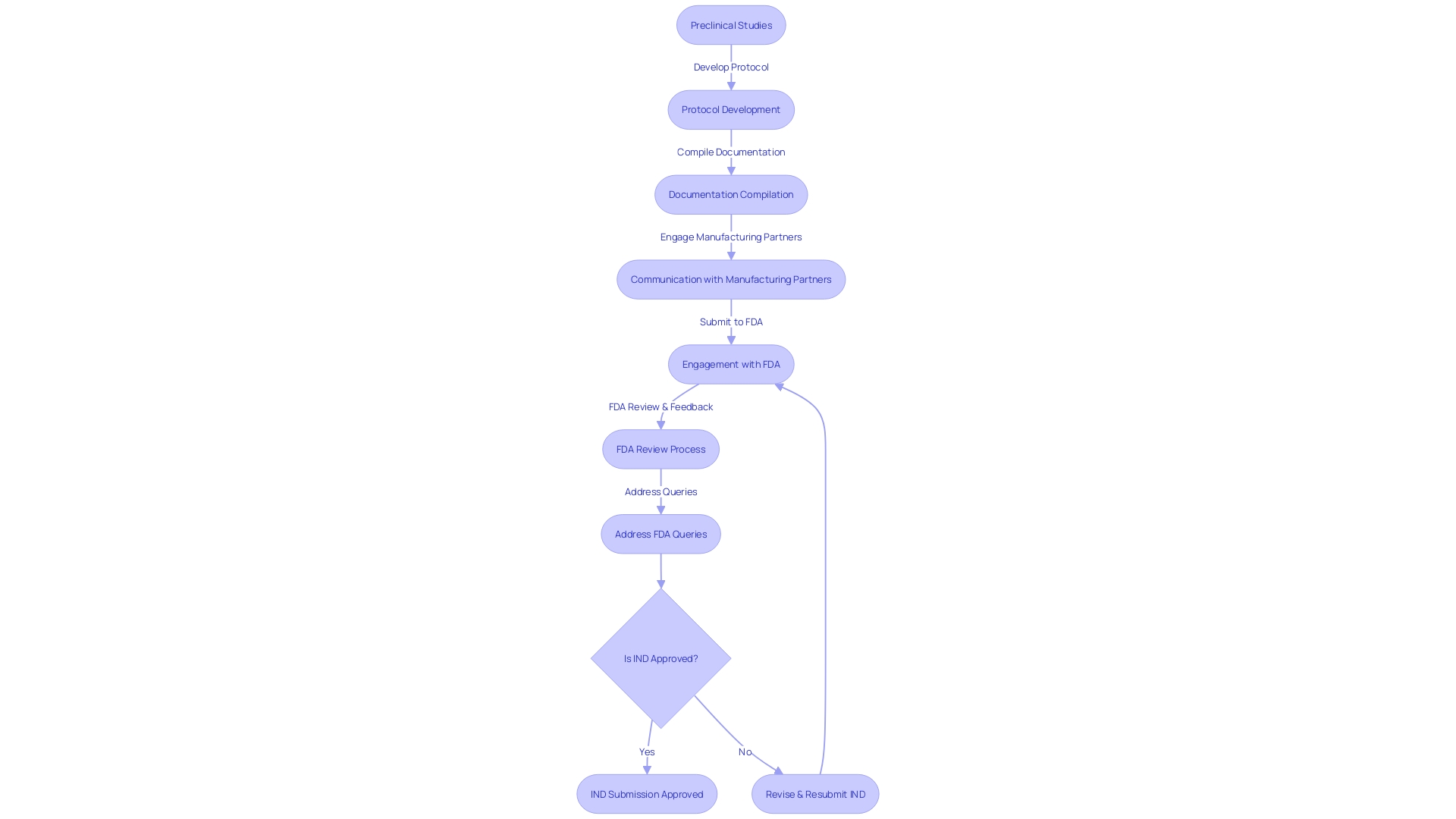
Firstly, initiating the IND submission process well in advance is essential. This foresight allows ample time for conducting preclinical studies, crafting a robust protocol, and compiling necessary documentation. Given the complexity of the task, involving regulatory consultants with a wealth of experience can offer invaluable guidance and potentially expedite the submission.
Organized and meticulous documentation is another cornerstone of an effective IND submission. It ensures that all necessary information is readily available and can be updated promptly. This level of organization is particularly critical given the dynamic nature of trials and regulatory requirements.
Furthermore, maintaining open and consistent communication with manufacturing partners is vital. It is through this collaboration that one can secure access to the comprehensive Chemistry, Manufacturing, and Controls (CMC) information required for the IND.
Lastly, being proactive and responsive to feedback from the Food and Drug Administration (FDA) is of paramount importance. This interaction is not just a regulatory hurdle but a constructive dialogue that can refine and improve the IND submission.
These practices are not only theoretical but are mirrored in real-world applications, as seen in Intellia Therapeutics' approach to their program for NTLA-2001, intended for the treatment of transthyretin (ATTR) amyloidosis. Despite the potential risks and uncertainties that come with clinical trials and product development, their commitment to rigorous scientific investigation and collaboration highlights the importance of preparedness and adaptability in the face of regulatory feedback.
In essence, the success of an IND submission is not solely in its approval but also in the robust preparation and proactive engagement throughout the process.
Conclusion
In conclusion, the IND application is a critical step in the journey from conception to approval of new medical therapies. It represents a formal request to the FDA for permission to initiate human trials and is grounded in a robust collection of preclinical data. The FDA's guidance and expertise are instrumental in this process, providing drug developers with necessary guidance on study design and data requirements.
The components of an IND application, such as the Investigator's Brochure, CMC information, preclinical data, clinical trial protocol, and informed consent forms, ensure a thorough evaluation of the investigational product and the planned clinical trial. Preparing for IND submission involves conducting preclinical studies, crafting a robust protocol, and compiling necessary documentation.
The IND submission process entails meticulous review by the FDA to confirm adherence to ethical and regulatory requirements. It is crucial for applicants to understand the gravity of this process and maintain transparency. Adherence to FDA standards is evident in various areas, such as regulatory requirements for prescription drug advertisements.
Lessons learned and best practices for IND submission include initiating the process well in advance, maintaining organized documentation, and being proactive and responsive to FDA feedback. By following these practices, drug developers can navigate the complex regulatory landscape and bring safe and effective therapies to patients.
In essence, the success of an IND submission lies not only in its approval but also in the robust preparation and proactive engagement throughout the process. It is through this comprehensive approach that new medical therapies can be brought to market, ensuring the safety and efficacy of treatments for patients.




