Introduction
Ensuring the safety, efficacy, and quality of medical devices is paramount in the highly regulated field of medical device manufacturing. Central to achieving these objectives is the rigorous process of validation, which meticulously assesses and confirms that manufacturing processes consistently produce products meeting predefined specifications and quality standards. This article delves into the critical stages of process validation—Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ)—and their indispensable role in maintaining product integrity and regulatory compliance.
The discussion will explore the significance of IQ, OQ, and PQ in the validation framework, detailing how each phase contributes to the overall validation process. Additionally, it will highlight the regulatory requirements set forth by authorities like the FDA and the European Union, underscoring the importance of adherence to these stringent guidelines. The benefits of implementing robust validation protocols will be examined, alongside best practices and common challenges faced during execution.
Throughout, the emphasis will be on how these validation steps not only ensure compliance but also enhance operational efficiency, reduce risks of nonconformance, and ultimately safeguard patient health. By understanding and effectively implementing IQ, OQ, and PQ protocols, manufacturers can uphold the highest standards of quality and safety in medical device production.
Understanding Process Validation and Its Importance
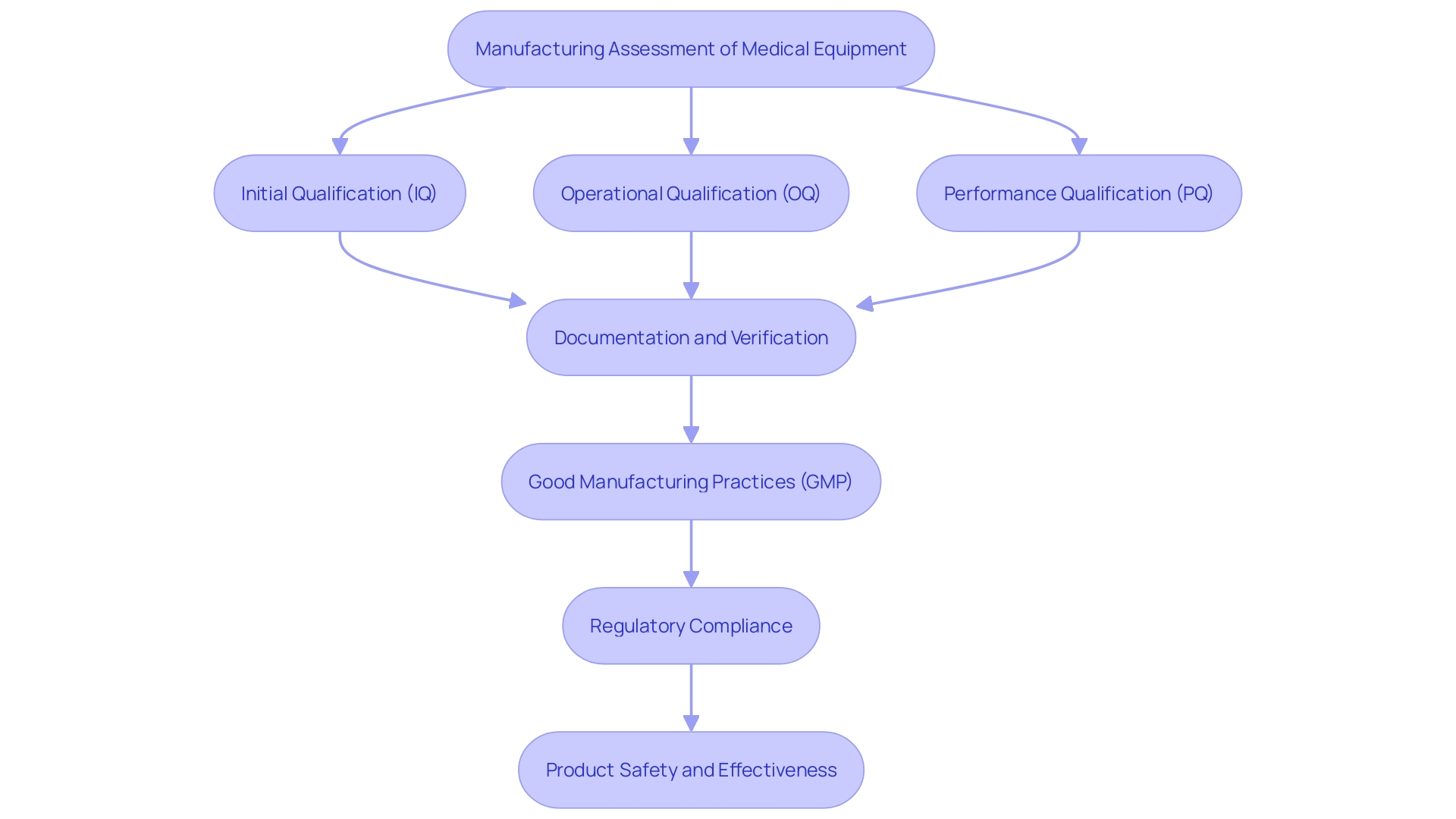
'Manufacturing assessment is an essential element in the lifespan of medical equipment, guaranteeing that production methods reliably produce items that satisfy established criteria and excellence benchmarks.'. This careful method of verification minimizes the dangers linked to equipment malfunctions, greatly improving product quality and ensuring adherence to regulations. Regulatory bodies, including the FDA and the European Union, have put in place strict guidelines for procedure confirmation to ensure that all manufacturing stages yield dependable and safe results.
In the strictly controlled setting of medical equipment production, procedure verification requires comprehensive documentation and meticulous focus on each stage, from initial qualification (IQ) and operational qualification (OQ) to performance qualification (PQ). This thorough assessment method goes beyond the physical creation of devices to encompass software systems, such as barcode labeling, to ensure that they operate properly, fulfill requirements, and function safely.
Good manufacturing practice (GMP) frameworks support this verification method, ensuring consistent excellence and compliance with global benchmarks such as ISO 13485. Despite the challenges posed by rigorous regulatory requirements, which can sometimes impede efficiency and innovation, the ultimate goal of procedure confirmation remains clear: to demonstrate that products are safe, effective, and suitable for their intended use, thereby safeguarding patient health and ensuring market approval.
What are IQ, OQ, and PQ?
IQ, OQ, and PQ are essential stages in the assessment of medical equipment, guaranteeing that each item complies with rigorous standards and regulatory criteria. Installation Qualification (IQ) confirms that equipment and systems are installed correctly according to predefined specifications, safeguarding the initial setup's integrity. Operational Qualification (OQ) tests the equipment's performance across its specified operating ranges, ensuring it functions as intended under various conditions. Performance Qualification (PQ) evaluates the system's performance under normal operating conditions, verifying that it consistently meets quality requirements. 'These verification steps are vital for maintaining the reliability and safety of medical devices throughout their lifecycle, from design and manufacturing to post-market surveillance.'.
Installation Qualification (IQ): Ensuring Correct Installation
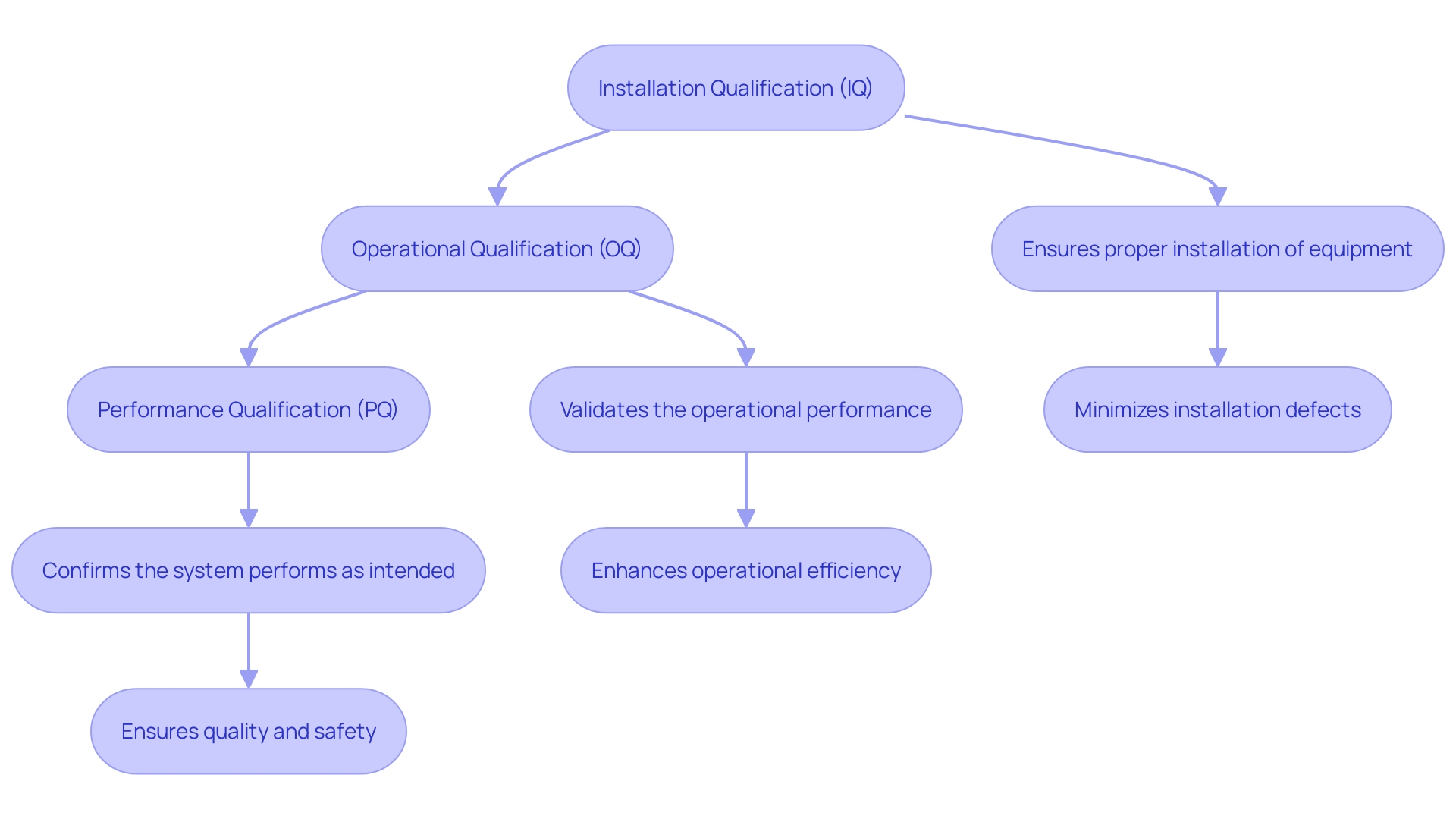
Installation Qualification (IQ) involves a comprehensive verification procedure to ensure that all equipment, systems, and components are installed and configured correctly, aligning with regulatory standards. This phase includes meticulous checks of documentation, adherence to installation procedures, and confirmation that operational parameters meet specified requirements. The main purpose of IQ is to create a strong basis for later assessment stages, ensuring that the environment and equipment are appropriate for their intended application.
Effective IQ is essential in highly regulated sectors such as medical device production, pharmaceuticals, and life sciences, where regulatory bodies like the FDA and the European Union require rigorous procedure verification. This validation provides documented evidence that all steps in the manufacturing procedure, including software systems like barcode label design and printing, are tested and validated to ensure quality outputs.
'The significance of a rigorous IQ procedure is highlighted by industry specialists who stress the necessity for thorough analysis and clear presentation of clinical data to prevent inconsistencies across different documents.'. These inconsistencies can lead to a disconnection between conclusions and actual data, highlighting the necessity for alignment in data collection and analysis strategies. Consequently, a well-executed IQ process not only ensures compliance but also enhances the reliability and safety of medical instruments, ultimately improving patient outcomes.
Operational Qualification (OQ): Verifying Equipment Operation
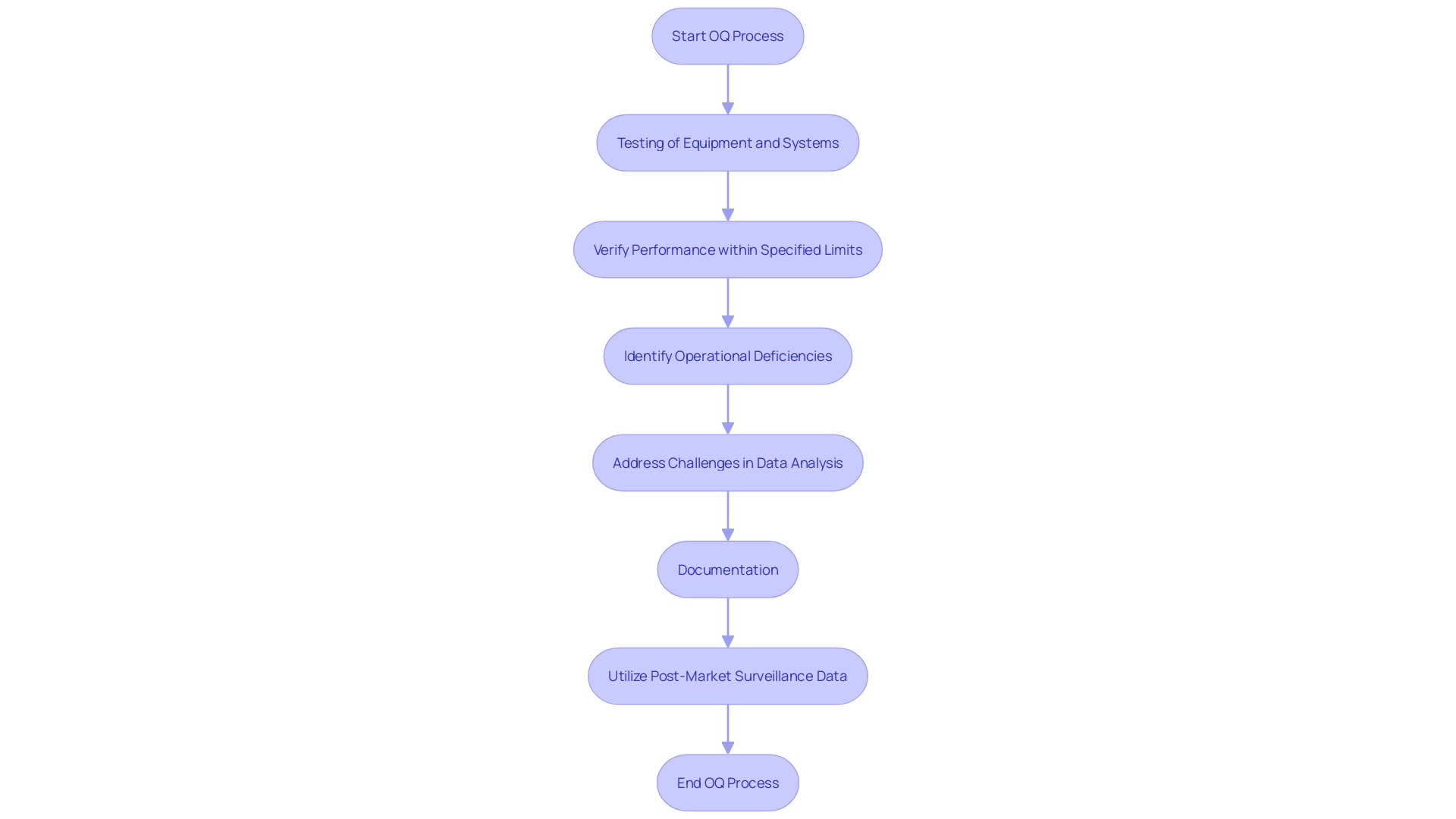
Operational Qualification (OQ) involves rigorous testing of equipment and systems to verify their performance within specified limits. This phase ensures the equipment's reliability and functional integrity across a range of conditions by assessing its response to various inputs. Identifying operational deficiencies during OQ is crucial as these can significantly impact product quality.
A common challenge in this phase is the meticulous analysis and presentation of data. Inconsistent documentation and lack of detailed analysis often lead to discrepancies between the actual data and the conclusions drawn. This is observed across several documents like clinical evaluation plans, reports, and risk management files, highlighting a need for alignment in data collection and analysis strategies.
Furthermore, post-market surveillance data, which is critical for comprehensive clinical evaluations, is frequently underutilized. This data, both from similar and the company's own products, should be analyzed with the same rigor as primary data to provide a thorough understanding of a device's performance and safety in real-world settings.
Performance Qualification (PQ): Validating Process Stability
Performance Qualification (PQ) is a critical phase in ensuring that the manufacturing method is not only effective but also consistent under typical operational conditions. This stage involves carrying out the procedure using actual or simulated production materials to verify that the output consistently meets the predefined specifications. By subjecting the method to real-world conditions, PQ confirms its stability and reproducibility. This careful method guarantees that the production sequence can consistently create items that conform to strict excellence criteria.
'Henry Ford encapsulated the essence of excellence with his statement, “Excellence means doing it right when no one is looking.” This principle is crucial during the PQ phase, where the commitment to superiority ensures the products' integrity and reliability.'. 'The manufacturing process must be designed, fabricated, tested, and adjusted to meet these high standards, regardless of the complexity or variety of the medical products being produced.'.
In the intricate world of medical device manufacturing, precision and excellence are paramount. Each component must be precisely measured and inspected to meet exact specifications, ensuring they function correctly within the human body. This involves scanning multiple parts or long components, detecting minute defects, and providing accurate feedback for necessary adjustments.
Moreover, the integration of digital quality management systems is becoming increasingly important. These systems help streamline the PQ procedure by providing a clear roadmap and phased implementation plan, which builds upon the success of previous digital transitions. This approach guarantees a solid base for future activities and aligns efforts toward achieving defined objectives and realistic goals.
Regulatory Requirements and Standards for IQ, OQ, PQ
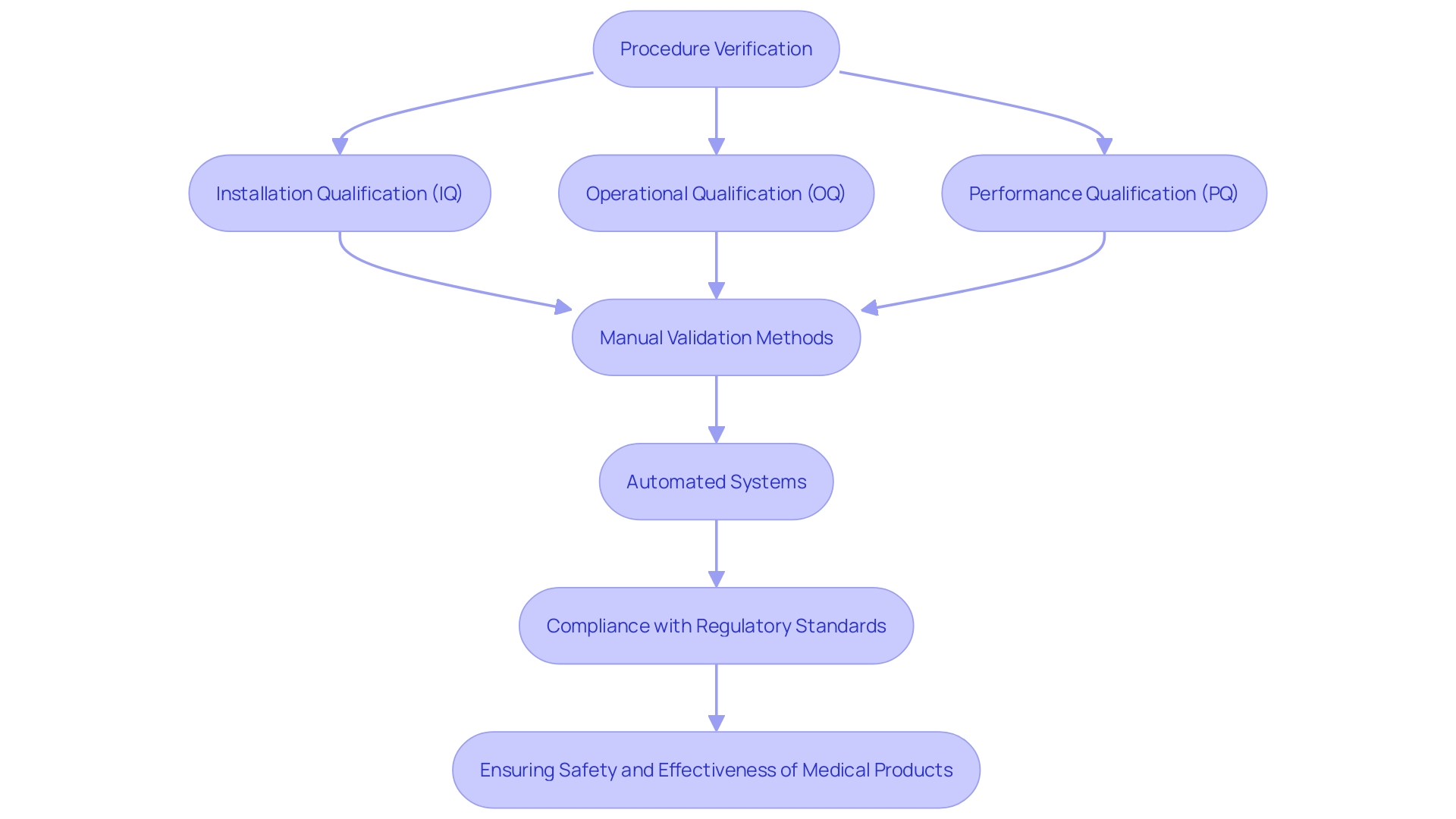
Regulatory bodies, such as the FDA and ISO, have established thorough guidelines for procedure verification, which encompass Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ). These stringent regulations require that manufacturers show the strength and adherence of their testing procedures with industry standards. The FDA, in particular, is pushing for a transition from manual validation methods—such as paper-based scripts and screenshots—to more automated systems using computer software. This change not only guarantees greater precision and productivity but also corresponds with the FDA's focus on improving the safety, effectiveness, and standard of medical instruments. Following these criteria is essential for producers to provide medical products that are safe, effective, and of superior standard, ultimately resulting in improved patient results.
Benefits of Implementing IQ, OQ, PQ in Medical Device Manufacturing
Implementing IQ (Installation Qualification), OQ (Operational Qualification), and PQ (Performance Qualification) offers numerous advantages that significantly enhance the quality and safety of medical instruments. These validation methods are essential in guaranteeing that all equipment and procedures adhere to strict regulatory standards, thereby minimizing the risk of defects and nonconformance issues.
In the meticulous domain of medical equipment manufacturing, where precision and adherence to specifications are paramount, IQ, OQ, and PQ play vital roles. For instance, these processes help to identify and rectify potential errors early in the production cycle, thus preventing costly recalls and ensuring product reliability. This proactive approach is especially important given the complex nature of medical devices, which range from simple bandages to sophisticated MRI machines and pacemakers.
Moreover, adhering to these validation protocols can significantly improve operational efficiency. By rigorously testing and validating each phase of production, manufacturers can detect and address issues such as human error or deviations from work instructions, which are common causes of nonconformance. 'This not only improves product standards but also simplifies the production workflow, resulting in better resource use and minimized downtime.'.
'The significance of these verification procedures is further emphasized by the push from regulatory organizations like the FDA, which promote the use of automated checking techniques over traditional manual methods.'. This shift towards automation not only minimizes the risk of human error but also ensures more consistent and reliable results.
In an industry where the consequences of nonconformance can be severe, maintaining robust IQ, OQ, and PQ protocols is not just about compliance; it's about safeguarding patient health and ensuring the highest standards of product quality and safety.
How to Execute IQ, OQ, PQ Protocols Effectively
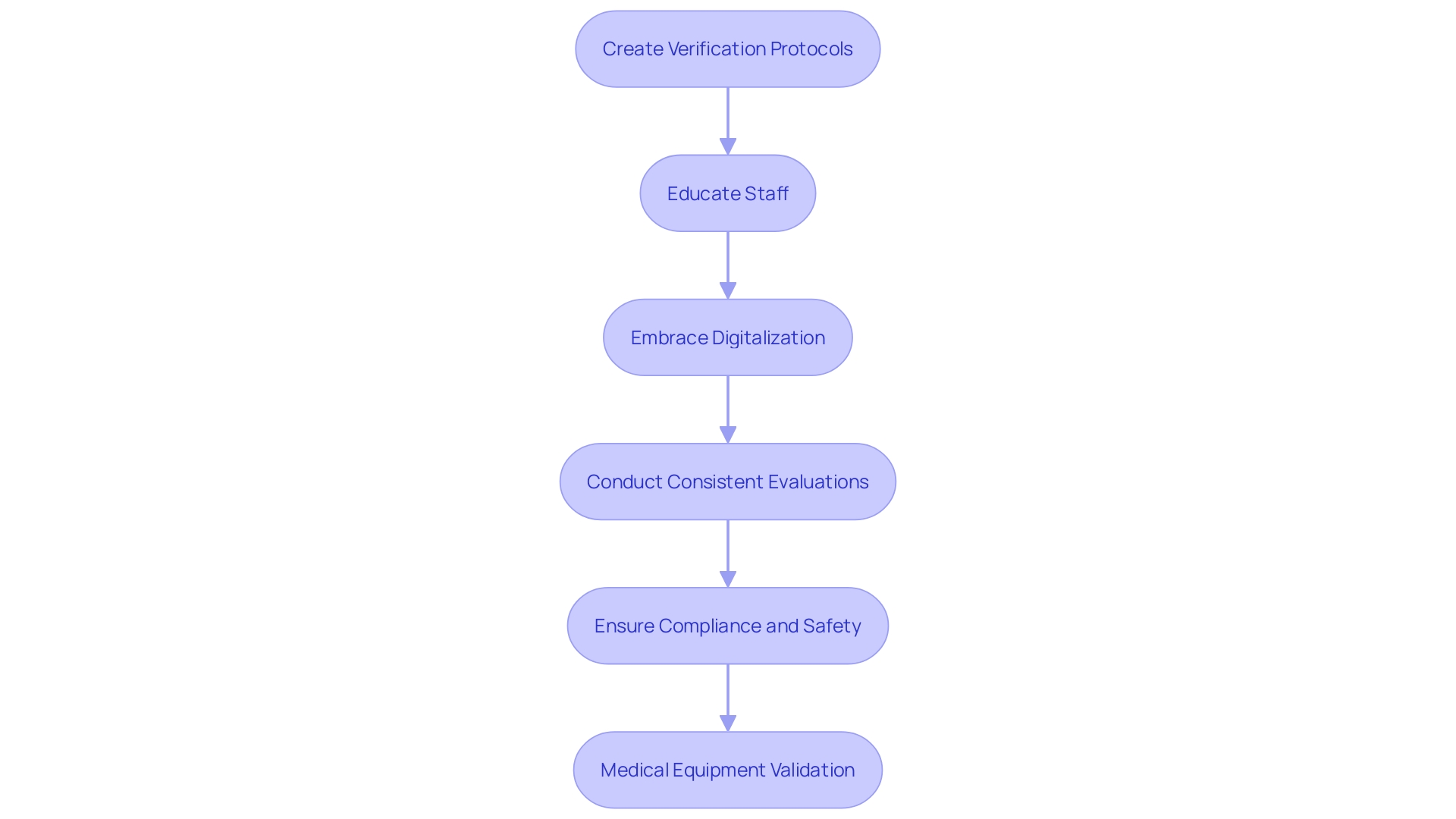
Executing IQ, OQ, and PQ protocols effectively necessitates meticulous planning and comprehensive documentation. Manufacturers should create comprehensive verification protocols that clearly outline objectives, methods, and acceptance criteria. Educating staff engaged in the verification task is crucial to guarantee uniformity and precision. Moreover, embracing digitalization can enhance the efficiency and reliability of these processes. Many medical equipment companies still depend on manual validation methods, but regulatory bodies like the FDA promote a more automated approach, which can reduce potential errors and streamline compliance efforts. Consistent evaluations and revisions to protocols, guided by both input and changing regulations, are essential for upholding compliance and guaranteeing the provision of safe and effective medical tools. Post-market surveillance data, often underutilized, should be rigorously analyzed to inform these updates and enhance risk management strategies.
Common Challenges and Best Practices for IQ, OQ, PQ Implementation
Implementing IQ, OQ, and PQ in the medical device industry presents several challenges, primarily due to inadequate documentation, lack of training, and insufficient resources. Regulatory bodies like the FDA and the European Union highlight the significance of validation in guaranteeing quality outputs. In highly regulated environments, every step in the manufacturing procedure must be meticulously documented and validated. This includes not only the production process but also related systems like barcode label design and printing.
To overcome these challenges, establishing clear communication among stakeholders is vital. Investing in comprehensive training programs ensures that all team members are well-versed in the necessary procedures and regulatory requirements. Creating a strong assessment plan that includes thorough documentation and frequent audits can help pinpoint areas for enhancement and ensure compliance with assessment requirements.
According to recent industry reports, gaining market approval and ensuring compliance with regulatory bodies remain top priorities for medical device leaders. The changing regulatory environment requires that companies adjust by utilizing more automated validation methods, as recommended by the FDA. Regular assessments and audits play a crucial role in maintaining compliance and improving overall process efficiency.
As pointed out by industry specialists, effective assurance of standards extends throughout the entire lifecycle of a medical instrument, from design to post-market monitoring. This systematic approach not only ensures compliance but also guarantees that medical devices consistently deliver safe and effective results. The complexity of global supply chains and rapid technological advancements further underscore the need for rigorous validation and quality assurance practices.
Conclusion
The importance of process validation in medical device manufacturing cannot be overstated. Through the rigorous stages of Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ), manufacturers ensure that their products consistently meet the stringent quality and safety standards mandated by regulatory authorities such as the FDA and the European Union. Each phase plays a pivotal role in establishing a reliable manufacturing process, thereby safeguarding patient health and ensuring market compliance.
By implementing robust validation protocols, manufacturers not only enhance product quality but also improve operational efficiency. Early detection of potential issues minimizes the risk of costly recalls and facilitates better resource utilization. Moreover, the shift towards automated validation methods, as advocated by regulatory bodies, further streamlines compliance efforts and reduces the likelihood of human error.
Despite the challenges associated with documentation, training, and resource allocation, best practices can be employed to navigate these hurdles effectively. Clear communication, comprehensive training, and regular audits are essential for maintaining compliance and ensuring that all stakeholders are aligned with the regulatory requirements. Ultimately, a steadfast commitment to IQ, OQ, and PQ processes is crucial in delivering safe, effective, and high-quality medical devices that meet the needs of patients and healthcare providers alike.




