Introduction
Medical device development is a complex endeavor that requires expertise and adherence to regulatory standards. Collaborating with Contract Research Organizations (CROs) is crucial for navigating this intricate landscape efficiently. In this article, we explore the importance of collaboration between medical device companies and CROs, how it impacts the development and deployment of medical technologies, and its role in enhancing patient outcomes.
We also delve into a case study highlighting a successful collaboration between a medical device manufacturer and a specialized CRO in the development of an advanced cardiac monitoring device. Additionally, we address key challenges in medical device development and how CROs play a vital role in simplifying the process and accelerating the availability of critical medical innovations. Join us as we uncover the pivotal role of collaboration in the ever-evolving field of medical device development.
The Importance of Collaboration
Medical device development is a complex venture requiring multidisciplinary expertise and rigorous adherence to regulatory standards. Collaboration with Contract Research Organizations (CROs) is critical for medical device companies to navigate this intricate landscape efficiently.
Chris, a biomedical engineer with 13 years in the medical device space, has seen firsthand the significant impact of CROss in managing clinical studies for Class III devices. His experience as a Solutions Engineer at Greenlight Guru underscores the necessity of expertise in regulatory compliance, clinical trial design, data management, and statistical analysis—areas where CROs excel.
The convergence of medical device innovation and CROs' operational efficiency can be seen through the lens of Digital Clinical Trials (DCTs). DCTs, utilizing technologies such as ePRO, eCOA, and remote monitoring devices, have democratized participant engagement by allowing for remote data collection. For instance, a Zelta CRO client successfully conducted a Phase III cardiovascular trial using remote blood pressure monitoring devices.
This approach not only streamlined data collection from hundreds of participants but also significantly cut costs and improved trial efficiency.
Furthermore, advancements in medical imaging technologies, like the CardiAQ system, highlight the evolution of medical devices. With high-performance sensors coupled with AI algorithms, the Cardiac device facilitates swift and precise detection of cardiac anomalies, enhancing early diagnosis and treatment. Its portability and ease of operation without specialized installation make it a practical choice for point-of-care use.
Business development leaders in healthcare, with decades of experience, recognize that successful medical device companies often culminate in acquisitions, IPOs, strategic alliances, or partnerships. It's not just about the innovation but also the strategic business decisions that drive a company towards a successful market presence.
In line with this, selecting a medical device development partner requires careful consideration of their full-service spectrum capabilities. An ideal partner brings to the table a comprehensive understanding of the regulatory landscape, market dynamics, and technical expertise, ensuring a seamless transition through development phases.
The medical device sector is diverse, ranging from simple consumer products to complex systems like MRI machines and pacemakers. The ongoing technological evolution in this field continues to introduce cutting-edge solutions, such as the use of materials science, bioengineering, and information technology.
The ideal medical device development trajectory is encapsulated by a quote from Dr. Thomas Fogarty, "An idea, by itself, has no importance whatsoever; it is the implementation of that idea and its acceptance by others that brings benefit to our patients." This reflects the essential collaboration between innovators and the healthcare ecosystem to deliver value and advance patient care.
In summary, the collaboration between medical device companies and CROs is integral to the successful development and deployment of medical technologies. It is a strategic partnership that leverages CROs' expertise to fulfill regulatory requirements, design effective clinical trials, manage data, and analyze statistics, ultimately leading to innovative solutions that enhance patient outcomes.
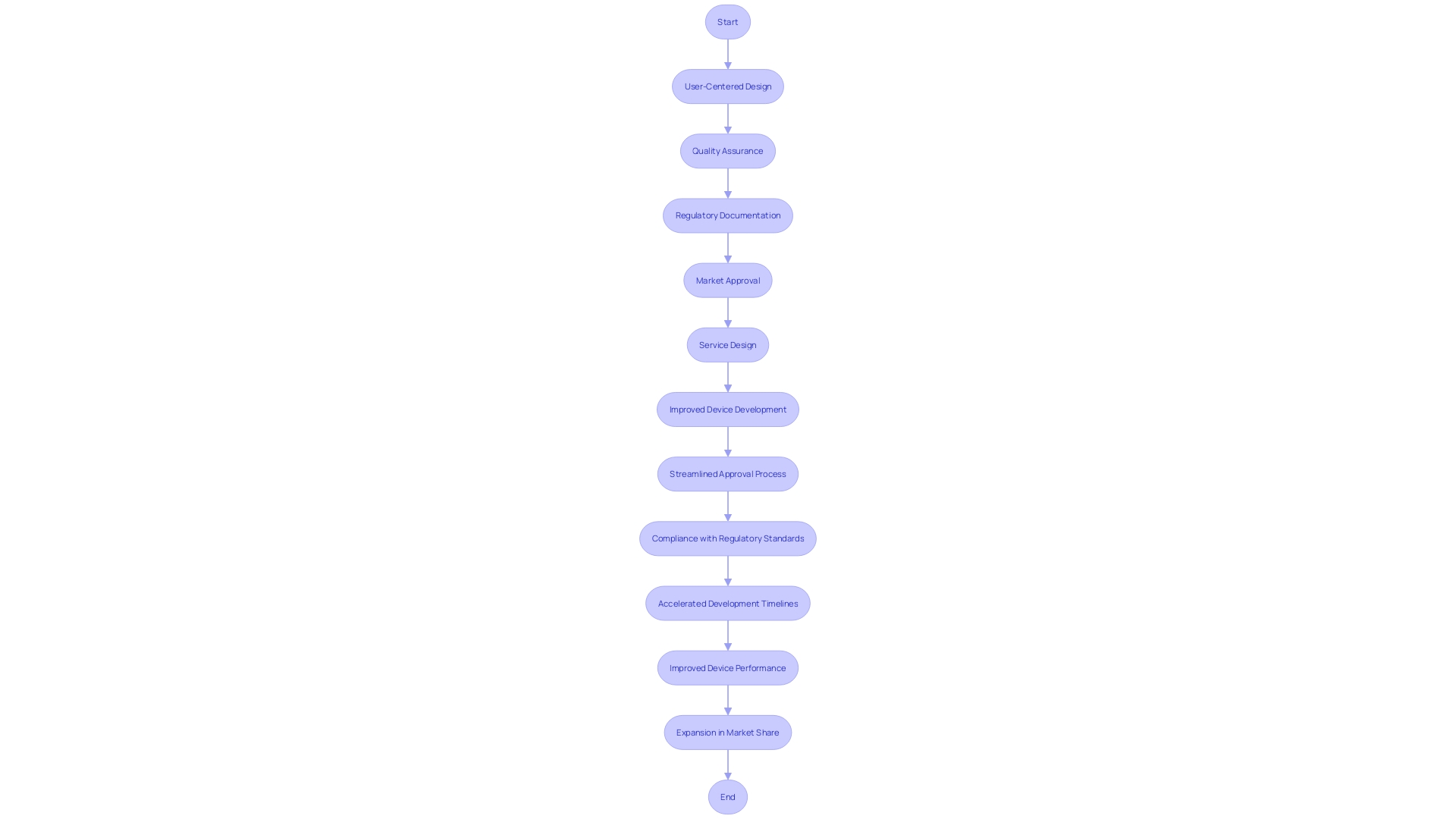
Case Study: Successful Collaboration with a Medical Device CRO
A collaboration between a medical device company and a Clinical Research Organization (CRO) can be pivotal in transforming medical innovations from concept to clinical application. Consider the partnership between a prominent medical device manufacturer, Company X, and a specialized CRO, Y. This partnership focused on the development of an advanced cardiac monitoring device.
Their collective expertise was crucial in managing the intricate regulatory requirements, which are particularly stringent for high-risk class three devices such as implantable pacemakers that sustain life. These devices, which account for around 10% of medical devices regulated by the FDA, undergo an extensive approval process to ensure safety and efficacy.
The collaborative efforts of Company X and CRO Y facilitated the smooth execution of clinical trials, comprehensive data analysis, and adherence to the rigorous standards set by regulatory bodies. This synergy was not only essential for obtaining FDA clearance but also for aligning with the European Medicines Agency (EMA) standards, which together with EU Member States oversee medical device regulation in Europe. Their success in securing regulatory approval has been a significant milestone in the cardiac health domain, offering a revolutionary approach to diagnosing and managing cardiac conditions.
The significance of this collaboration is underscored by the evolving landscape of medical device governance, where streamlined regulatory pathways and industry-regulator partnerships are increasingly encouraged. This is particularly evident as the medical industry seeks to address urgent unmet medical needs, which has been highlighted by the COVID-19 pandemic. The drive towards digital health and personalized medicine further emphasizes the importance of such partnerships.
The case of Company X and CRO Y exemplifies the impactful convergence of medical device innovation and specialized regulatory strategy, setting a benchmark for future advancements in the healthcare sector and the enhancement of patient outcomes through technological breakthroughs.
Addressing Key Challenges
The realm of medical device development is marked by a unique blend of diversity, ranging from simple consumables to sophisticated life-supporting machinery. With over 10,000 types of medical devices recognized by the World Health Organization, the sector encompasses a vast array of technologies including materials science, bioengineering, and software development. Clinical Research Organizations (CROs) are pivotal in navigating this multifaceted domain, particularly when confronting the rigors of regulatory compliance.
In the United States, the FDA classifies devices into three categories, with class three devices such as pacemakers undergoing the most stringent review process. Despite the inherent complexity, recent initiatives have sought to streamline regulatory pathways, a movement accentuated during the COVID-19 pandemic to hasten the availability of critical medical innovations.
CROs leverage their expertise to simplify the development process, advising on essential versus non-critical product features and embracing User Experience (UX) design to enhance both usability and clinician satisfaction. By focusing on the essential elements that ensure safety and performance, and deferring ancillary features to post-market updates, CROss help medical device companies reduce the time and effort required to bring new products to market.
Moreover, CROs contribute significantly to the efficient design and execution of studies by optimizing resource allocation and utilizing their networks to expedite patient recruitment and data collection. This is instrumental in mitigating time limitations and ensuring that medical devices reach the market and patients more swiftly, ultimately fostering advancements in healthcare delivery and patient outcomes.
Conclusion
Collaboration between medical device companies and Contract Research Organizations (CROs) is crucial for navigating the complex landscape of medical device development. CROs excel in regulatory compliance, clinical trial design, and data management, essential for success in this field. The convergence of innovation and CROs' efficiency is evident in Digital Clinical Trials (DCTs), streamlining data collection and improving trial efficiency.
Successful medical device companies often culminate in acquisitions, IPOs, or partnerships. Selecting the right development partner requires considering their capabilities in regulatory understanding, market dynamics, and technical expertise. Collaboration between innovators and the healthcare ecosystem advances patient care.
A case study of a successful collaboration between a medical device manufacturer and a specialized CRO in developing an advanced cardiac monitoring device sets a benchmark for future advancements. This partnership facilitated smooth trials, data analysis, and adherence to rigorous standards.
CROs also address key challenges in medical device development. They simplify the process by advising on essential product features, optimizing resources, and expediting patient recruitment and data collection. This accelerates the availability of critical medical innovations, enhancing patient outcomes.
In conclusion, collaboration between medical device companies and CROs is integral to successful development. It leverages CROs' expertise in regulatory compliance, trial design, and data management to deliver innovative solutions that enhance patient outcomes.




