Introduction
In the highly regulated and rapidly advancing field of medical devices, maintaining rigorous quality assurance (QA) is crucial to ensuring product safety and efficacy. This article delves into the key components of medical device QA, emphasizing stringent design controls, comprehensive documentation, standardized testing protocols, and continuous monitoring. It further explores the critical role of regulatory compliance, highlighting the stringent requirements set by bodies such as the FDA and EMA.
Additionally, the article examines the pivotal role of risk management in mitigating potential hazards throughout the device lifecycle. Finally, it addresses the challenges and future directions in medical device QA, focusing on emerging technologies, global regulatory harmonization, and the need for innovative methodologies to navigate the evolving landscape.
Key Components of Medical Device Quality Assurance
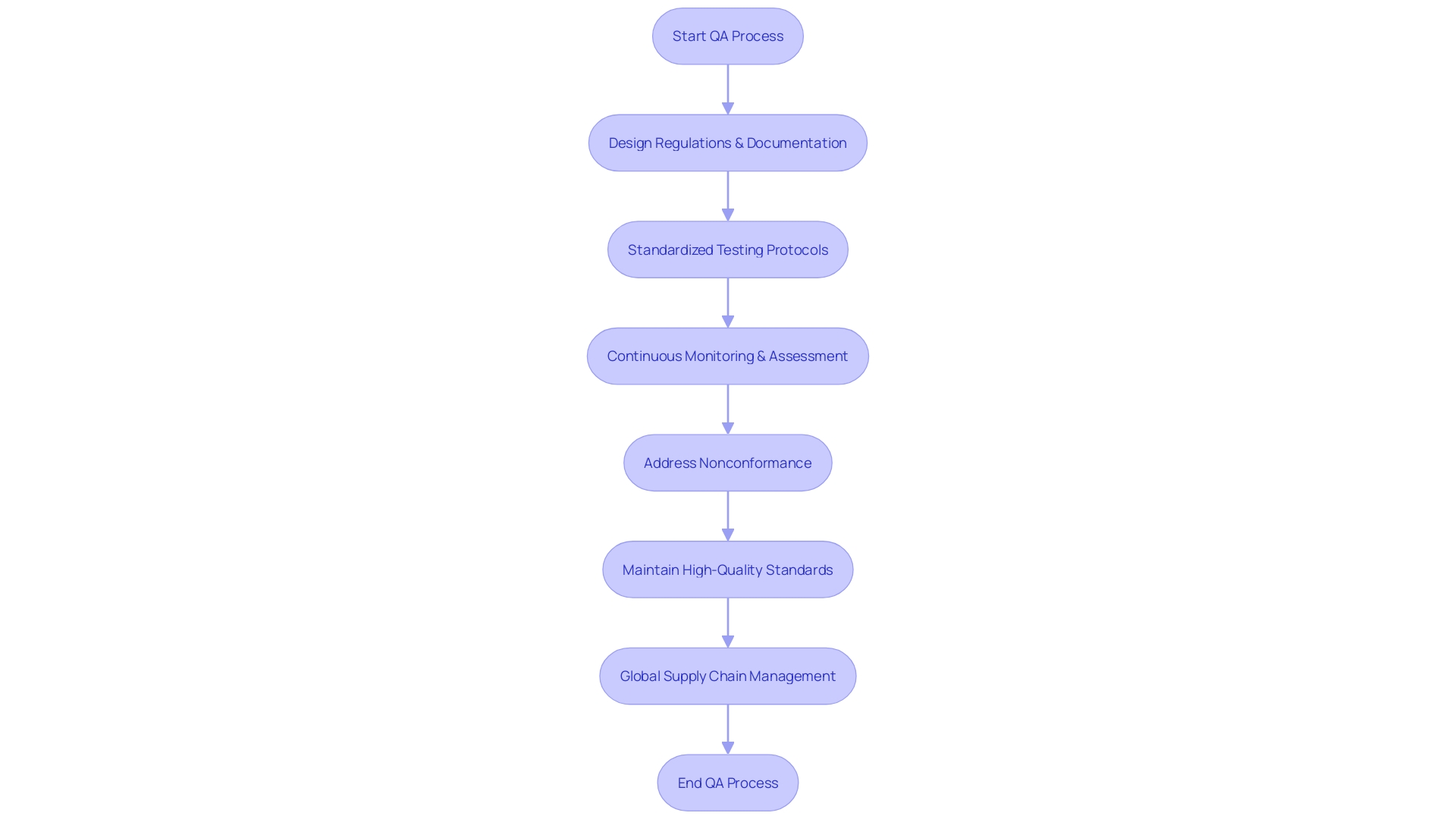
Quality assurance (QA) in medical product development involves a comprehensive approach focused on maintaining high standards and regulatory compliance throughout the product lifecycle. This process begins with rigorous design regulations, ensuring that each item meets predefined requirements and addresses potential risks effectively. Thorough documentation practices play a crucial role in this, as they allow for meticulous tracking and validation of each step in the development process.
Standardized testing protocols are also essential, as they verify that the devices perform safely and effectively under various conditions. This includes human factors engineering and usability engineering plans, which place users at the center of the design process to ensure that their needs are met in real-world settings.
Effective QA processes extend to continuous monitoring and assessment, which are vital for ongoing compliance with regulatory standards. 'This is especially important considering the varied nature of healthcare devices—ranging from simple consumer products to complex systems like MRI machines and pacemakers—and the rapid pace of technological advancements in the MedTech field.'.
Moreover, the global supply chain adds another layer of complexity, making it imperative for companies to maintain high-quality standards across all suppliers. Nonconformance at any stage, whether due to design defects, manufacturing errors, or user mistakes, must be documented and addressed promptly to prevent any compromise in product safety or efficacy.
Including these QA elements guarantees that healthcare products not only adhere to strict regulations but also reliably provide safe and effective outcomes. 'As compliance requirements continue to change, remaining flexible and responsive in quality management practices is crucial for success in the healthcare equipment sector.'.
Regulatory Compliance for Medical Devices
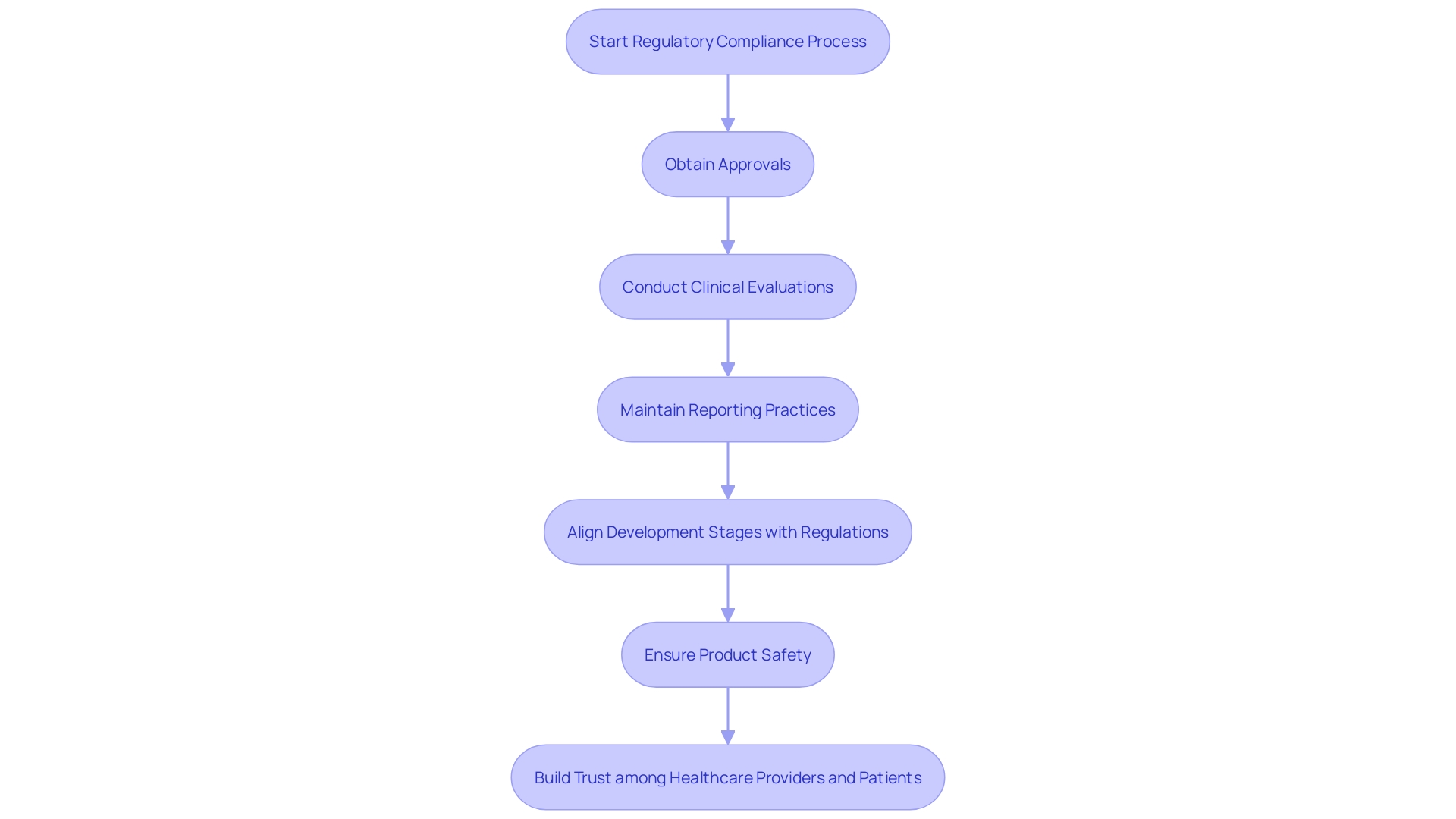
'Regulatory compliance is a cornerstone of the healthcare equipment sector, governed by stringent national and international standards.'. Organizations must adhere to guidelines established by oversight bodies such as the FDA in the United States and the European Medicines Agency (EMA) in Europe. In Europe, the Medical Equipment Regulation (MDR) has transformed the legal framework, necessitating all new health-related tools to adhere to updated standards and imposing a transition period for current products to be recertified. This transition can be complex, but essential for market access.
Compliance involves several critical steps, including obtaining necessary approvals before market entry, conducting comprehensive clinical evaluations, and maintaining rigorous reporting practices. 'According to a study, 47% of healthcare product leaders identified ensuring adherence to regulatory bodies as a top priority, highlighting the increasing challenges due to tougher legislation and a growing volume of literature to review.'.
'These regulations extend to aspects such as pre-market research, development and clinical evidence, and the post-market safety, efficacy, and performance of healthcare products.'. Furthermore, items that merge therapeutic substances and healthcare apparatus are governed by relevant systems based on their main method of operation, introducing an extra level of intricacy.
Ensuring all development stages align with these regulations is not merely about upholding product safety; it also builds trust among healthcare providers and patients. 'As Dídac López, a QA Principal at ERNI, emphasizes, a comprehensive understanding of the regulatory landscape and thorough testing are crucial for the successful certification and market entry of healthcare products.'. This comprehensive method guarantees that health tools are both secure and efficient, ultimately cultivating trust in the healthcare sector.
The Role of Risk Management in Medical Device QA
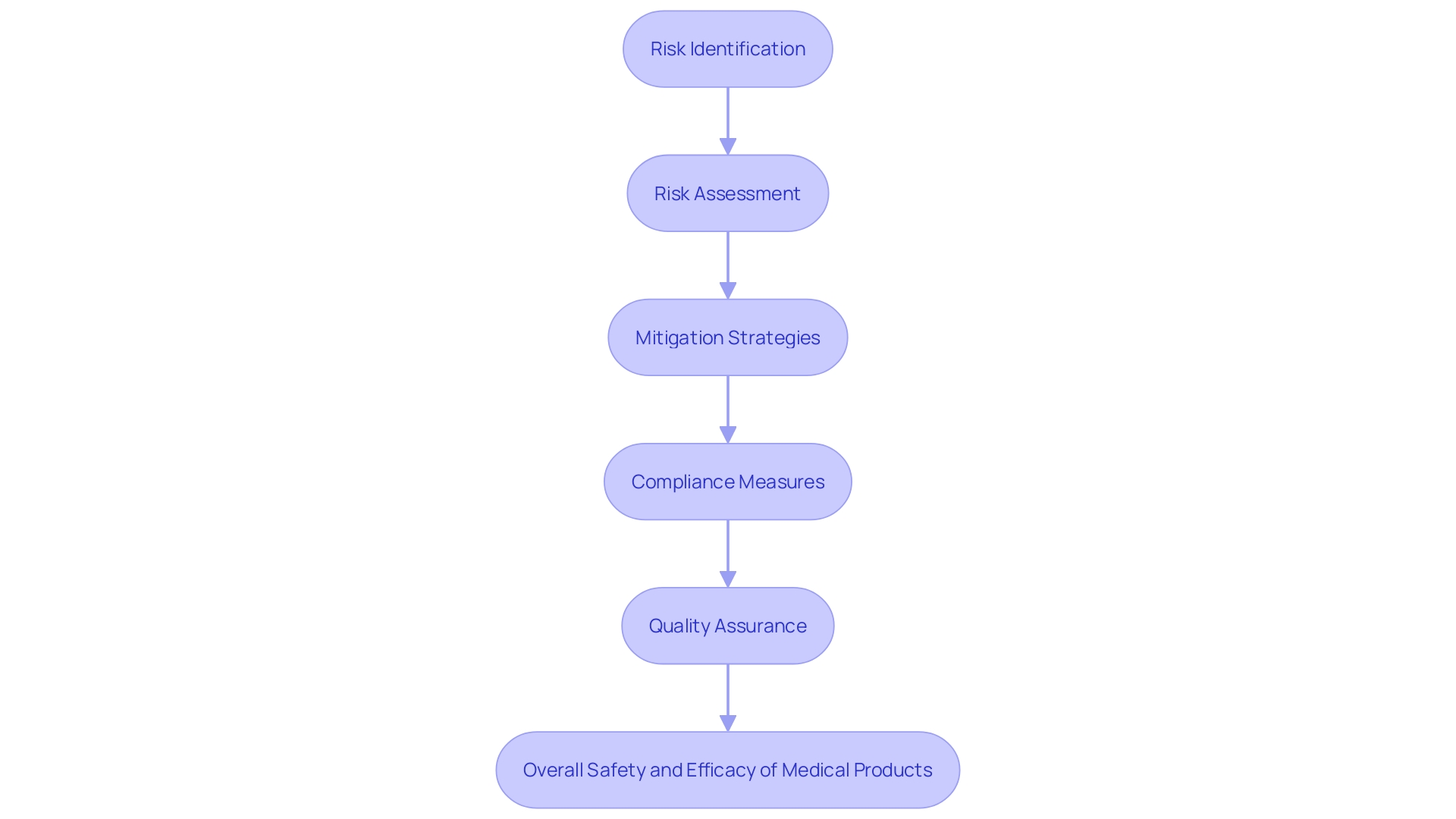
Risk management is a cornerstone of quality assurance in medical equipment projects, pivotal in ensuring product safety and efficacy. This complex procedure entails recognizing, examining, and reducing possible threats that could impact the apparatus during its lifecycle.
Incorporating management principles early in the development stages is essential. By adopting a proactive approach, teams can anticipate and address potential issues before they escalate. This innovative strategy not only reduces risks but also improves the overall quality of the medical product, ensuring adherence to standards and optimal patient outcomes.
For instance, the Quality Management System Requirements (QMSR) emphasize the significance of integrating hazard management into design, manufacturing, and post-market surveillance. This thorough integration assists manufacturers in recognizing and addressing challenges efficiently, resulting in the creation of high-quality products that comply with strict regulatory standards.
Professionals such as Bijan Elahi, who moved from aerospace to medical device safety management, emphasize the importance of clear and precise safety management practices. 'Elahi's work, which has educated over 10,000 professionals globally, emphasizes best practices and thorough methodologies for safety management.'. 'His insights clarify the complexities of management standards, providing practitioners with the tools they need to perform detailed analyses and maintain progress.'.
Moreover, the changing environment of compliance requirements, as emphasized by recent advances in systems like ArisGlobal's LifeSphere® Compliance platform, demonstrates the significance of being proactive in management practices. 'These platforms streamline compliance workflows, enhancing visibility and data quality, which is crucial for upholding adherence and efficiency in a rapidly changing environment.'.
In summary, efficient hazard management is crucial for guaranteeing the safety and quality of healthcare instruments. By adopting comprehensive risk evaluation methods and adhering to compliance standards, manufacturers can create products that are not only safe and effective but also meet the highest quality benchmarks, thereby safeguarding patient health and well-being.
Challenges and Future Directions in Medical Device Quality Assurance
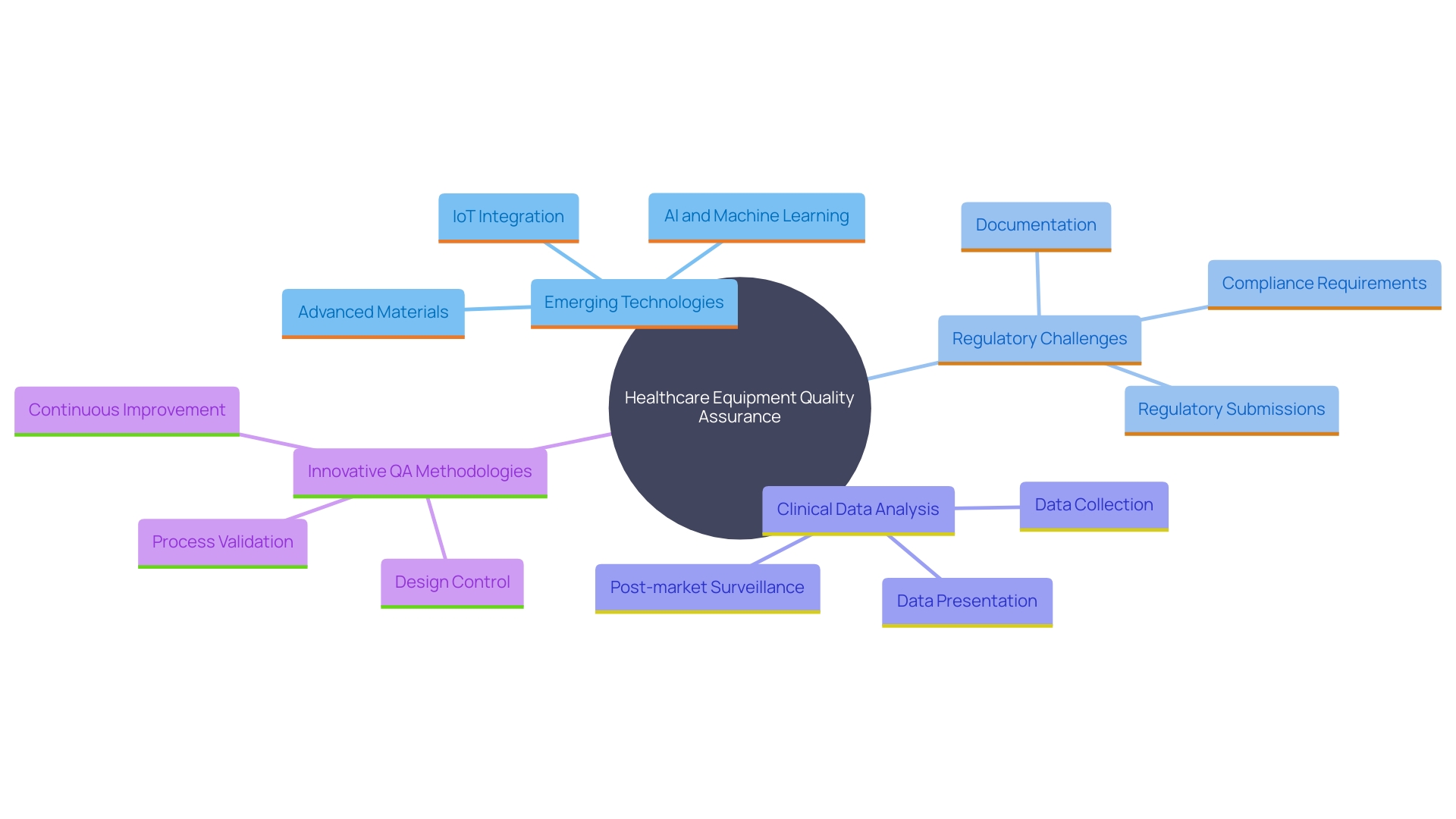
The terrain of healthcare equipment quality assurance is changing swiftly, presenting both obstacles and prospects. Emerging technologies, such as artificial intelligence and digital health solutions, introduce new complexities in QA processes. As Jennifer Mascioli-Tudor puts it, "A truly skilled quality and compliance professional needs to have excellent project management abilities." This highlights the necessity for adaptability and agility in building effective QA systems.
Furthermore, the harmonization of global regulations poses significant challenges for manufacturers striving to meet diverse compliance requirements. 'According to James Pink, a recognized technical expert, concentrating on the next generation of software that supports the safety and performance of healthcare instruments is essential for managing these regulatory challenges.'. The intricate nature of global medical device regulations, especially for Software as a Medical Device (SaMD), demands a deep understanding of regional nuances and compliance processes. The SaMD market is poised for significant growth, with a projected compound annual growth rate of approximately 52% from 2023 to 2028.
Additionally, Bijan Elahi, an expert in safety risk management, emphasizes the importance of meticulous analysis and presentation of clinical data. Post-market surveillance data, often underutilized, plays a crucial role in MedTech clinical evaluations and must be analyzed rigorously to ensure compliance and efficacy.
Looking forward, the industry must adopt innovative QA methodologies, enhance collaboration among stakeholders, and invest in training and resources. As the industry grapples with these challenges, improving efficiency in regulatory and safety document preparation remains a top priority. By leveraging advancements in technology and fostering a collaborative approach, the medical device sector can navigate the evolving landscape effectively and ensure the safety and performance of medical devices in global markets.
Conclusion
The significance of a robust approach to medical device quality assurance is clear, involving stringent design controls, thorough documentation, standardized testing, and continuous monitoring. These components are vital for regulatory compliance and ensuring high safety and efficacy standards throughout the product lifecycle.
Regulatory compliance is fundamental, governed by strict standards from authorities like the FDA and EMA. Recent regulations, such as the Medical Device Regulation (MDR) in Europe, highlight the need for a deep understanding of compliance protocols, fostering trust among healthcare providers and patients while ensuring safe market access.
Risk management is crucial in this context, focusing on the proactive identification and mitigation of potential hazards. By integrating risk management early in the development process, manufacturers can significantly enhance product quality and regulatory adherence, ultimately protecting patient health.
As the medical device landscape evolves with emerging technologies and global regulatory harmonization, the industry must adapt to these complexities. Emphasizing innovative methodologies, collaboration among stakeholders, and ongoing training will be essential for navigating this dynamic environment. This commitment to quality assurance not only ensures compliance but also reinforces the dedication to delivering safe and effective medical devices that meet the needs of healthcare providers and patients.




