Introduction
Serious Adverse Events (SAEs) hold critical importance in clinical trials, representing significant medical occurrences that could lead to severe or life-threatening conditions for participants. These events go beyond anticipated side effects and require prompt intervention. For example, a recent clinical trial involving lecanemab, aimed at treating Alzheimer's disease, resulted in a patient experiencing debilitating headaches and worsening cognitive impairment after receiving the active drug.
The consequences of SAEs range from prolonged hospitalization to disability and even death. Recognizing and understanding these events are pivotal for participant safety and evaluating the true risks and benefits associated with clinical research. Regulatory bodies, such as the FDA, emphasize data governance and participant safety, highlighting the growing recognition of the critical role that SAEs play in clinical trials.
This article explores the definition, examples, identification criteria, reporting requirements, and importance of SAE narratives in the context of clinical research.
Definition of Serious Adverse Events (SAEs)
Serious Adverse Events (SAEs) hold critical importance in clinical trials, as they represent significant medical occurrences that could lead to severe or life-threatening conditions for participants. These incidents go well beyond the realm of anticipated side effects and necessitate prompt intervention. A vivid illustration of an SAE can be drawn from a recent clinical trial involving lecanemab, aimed at treating Alzheimer's disease. Despite initial screenings showing no adverse effects, the administration of the active drug in the trial's extension phase led to the patient experiencing debilitating headaches and worsening cognitive impairment, highlighting the gravity such events can carry.
The consequences of SAEs are diverse, ranging from prolonged hospitalization to persistent or significant disability and even congenital anomalies or death. Recognizing and understanding these events are not just critical for participant safety but also pivotal in evaluating the true risks and benefits associated with clinical research.
Recent updates in clinical trial regulations emphasize the significance of data governance and participant safety. For instance, the FDA's final rule on direct-to-consumer prescription drug advertisements insists on presenting major side effects and contraindications in a transparent and understandable manner, ensuring that the public is well-informed. This reflects the broader commitment of regulatory bodies to protect participants and maintain the integrity of clinical data.
Moreover, the evolving guidelines on Good Clinical Practice (GCP) by organizations like the International Council for Harmonization (ICH) underscore the necessity for meticulous design and risk assessment in trials, prioritizing activities central to achieving trial objectives and safeguarding participant well-being. These advancements in regulatory guidelines serve as a testament to the growing recognition of the critical role that SAEs play in clinical research.
It's imperative for those conducting and overseeing clinical trials to maintain a comprehensive grasp of what constitutes an SAE and related terms. This knowledge is foundational in ensuring ethical conduct, securing the rights and safety of participants, and bolstering the credibility and reliability of research outcomes.
Examples of SAEs
Clinical trials are essential for advancing medical science, but they can also present risks to participants through Serious Adverse Events (SAEs). SAEs are defined as any untoward medical occurrences that result in death, are life-threatening, require hospitalization or prolongation of existing hospitalization, result in persistent or significant disability/incapacity, or lead to a congenital anomaly/birth defect. In a recent example, a patient in a trial for lecanemab experienced severe headaches and worsening memory impairment after receiving the treatment, highlighting the critical nature of monitoring and reporting these events.
The implications of SAEs are profound, not just for patient safety, but also for the integrity of the trial results and the future of the therapeutic under investigation. This is reflected in the findings of a study by Kelly Cobey and colleagues, which noted the lack of public records for many trial results, emphasizing the importance of transparency in clinical research. The study also revealed a significant gap in trial registration and reporting, with only 59% of trials registered prior to participant enrollment and 32% of studies failing to report results.
Moreover, leading biopharmaceutical companies like Pfizer recognize the importance of safety in clinical research, as they strive to set quality and safety standards in the development of healthcare products. It is through rigorous safety monitoring and adherence to protocols that organizations can fulfill their commitment to advancing treatment options while protecting those who participate in clinical trials. The role of systems like the Vaccine Adverse Event Reporting System (VAERS) is critical in this context, as they help identify safety issues and contribute to the national vaccine safety system, underscoring the multifaceted approach needed to manage Sales effectively.
Criteria for Identifying SAEs
When delineating Serious Adverse Events (SAEs) from other types of adverse events in clinical trials, researchers adhere to a set of specific criteria. These criteria encompass the event's severity, the outcome's impact on the participant, the investigational product's causal relationship, and the necessity for medical intervention. This structured approach not only ensures the consistent and accurate identification of Sales but also aligns with regulatory expectations and supports the integrity of the clinical trial data.
For instance, reflecting on the complexity of cancer drug approvals, it's evident that the determination of Sales requires careful consideration and transparency. As discussed on the Plenary Session podcast, the implications of Sales are significant, influencing patient safety, the cost of healthcare, and the ethical standing of pharmaceutical companies. Similarly, the OCEANIC-AF trial showcases the importance of robust clinical trial design and the value of investigating new therapies through proper trials that focus on critical outcomes like survival and stroke prevention.
Publications like Medical Device News Magazine serve industry experts and patients alike by emphasizing the relevance of such data and the implications of SAEs in clinical trials. The commitment to testing new interventions in a controlled and rigorous manner, as seen in the OCEANIC-AF trial, highlights the significance of SAE reporting in advancing medical science and ensuring patient safety.
Reporting Requirements for SAEs
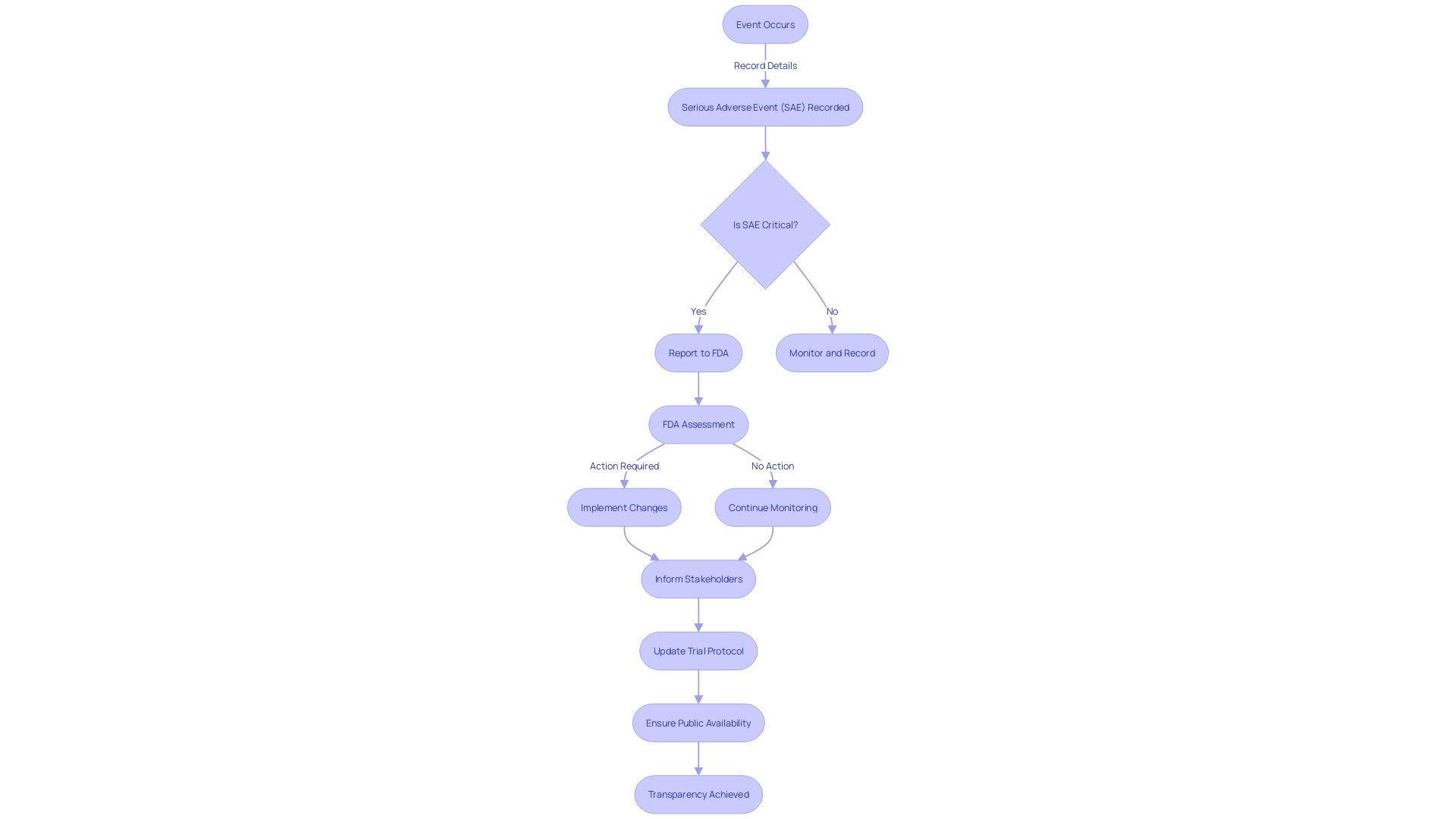
The meticulous documentation and reporting of Serious Adverse Events (SAEs) are pivotal in clinical trials, ensuring that the safety of participants is vigilantly monitored. Regulatory bodies, such as the FDA, mandate the precise recording of Sales, encompassing the event's specifics, the affected individual's medical background, the consequent actions, and the eventual outcomes. This stringent reporting is not a mere procedural formality; it is integral to evaluating the investigational product's benefit-risk balance and upholding the integrity of clinical research.
For example, clinical trials targeting transthyretin-mediated amyloidosis, a severe and life-threatening condition, underscore the gravity of SAE reporting. Participants, often managing multiple health issues, may experience a spectrum of side effects and new health challenges as part of the trial. The dedication of these individuals, who participate in the hope of advancing research for future generations, adds a profound ethical dimension to the accurate reporting and management of SAEs.
In this landscape, innovations such as AI and ML are being scrutinized by regulatory agencies to ensure they meet elevated compliance standards. The evolving guidelines, like the EU AI Act, reflect a commitment to balancing patient safety with technological progress.
Reflecting on the significance of SAE reporting, the FDA asserts the importance of systems like VAERS, which has been instrumental in identifying vaccine safety concerns. The proactive engagement of all stakeholders, including patients, healthcare professionals, and pharmaceutical companies like Pfizer, in reporting adverse events, is crucial for maintaining the safety and efficacy of medical interventions.
As we navigate the complexities of SAEs in clinical trials, it's clear that the overarching aim is to safeguard public health while fostering the development of groundbreaking treatments. This objective is at the heart of clinical research, driving the meticulous attention to detail that is vital for the advancement of medicine.
SAE Narratives: Importance and Structure
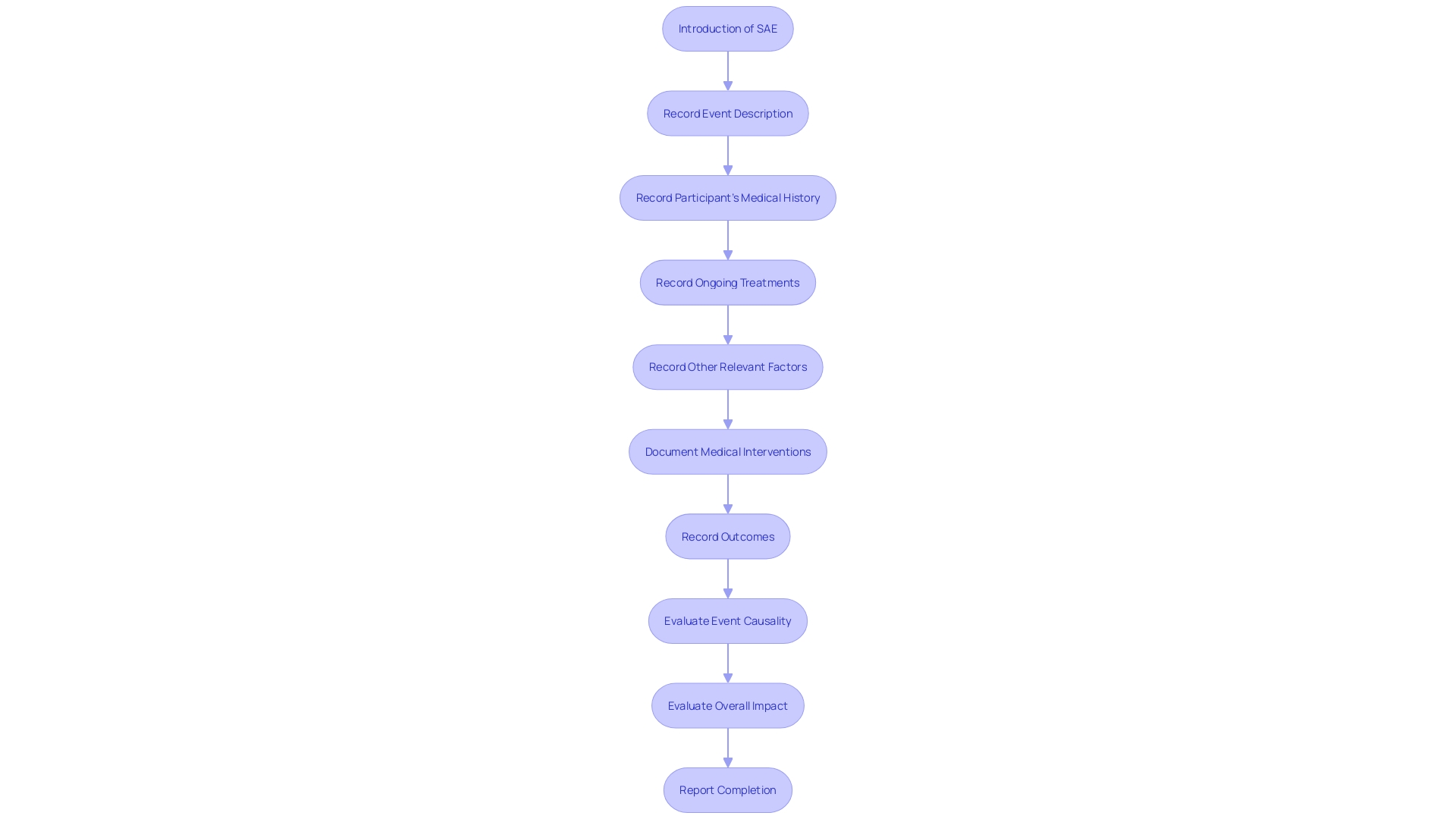
Within the scope of clinical trials, the documentation of Serious Adverse Events (SAEs) through comprehensive narratives is indispensable. These narratives meticulously record the sequence of the SAE from its inception through to its resolution, integrating details such as the participant's medical history, ongoing treatments, and other pertinent factors that could influence the event. The narrative structure is methodically organized, commencing with an introduction, followed by a detailed event description, the medical interventions undertaken, the outcomes, and culminating with a conclusion. The precision of these narratives is pivotal for the accurate evaluation of the event's causality and overall impact. Consistent and thorough reporting practices are fundamental, especially considering the complexities of clinical scenarios, such as the treatment of patients with multiple coronary lesions, where multiple stents or coronary bypass surgery may be considered. Adherence to consistent outcome measures and reporting guidelines ensures the reliability of the trial's findings, which is essential when interpreting the data and making informed decisions about patient care.
Conclusion
SAEs in clinical trials hold critical importance, going beyond anticipated side effects and requiring prompt intervention. Examples like the lecanemab trial highlight the gravity of SAEs, ranging from debilitating headaches to disability and death. Recognizing and understanding SAEs is pivotal for participant safety and evaluating risks and benefits.
Regulatory bodies emphasize data governance and participant safety, reflecting the growing recognition of SAEs' role in trials.
Criteria for identifying SAEs ensure consistent and accurate identification, aligning with regulatory expectations. Meticulous documentation and reporting are pivotal to monitor participant safety and uphold research integrity. SAE narratives play an indispensable role, documenting events from inception to resolution, ensuring precision and facilitating accurate evaluation.
In conclusion, SAEs are of utmost importance in clinical trials. Accurate identification, documentation, and reporting are crucial for participant safety and research credibility. Adhering to regulatory guidelines and maintaining meticulous reporting practices allow for the advancement of medicine while safeguarding participants.
Learn how bioaccess™ can help ensure participant safety in clinical trials.




