Introduction
The 510(k) clearance process is a critical step for medical device manufacturers seeking to bring new devices to the market. This process allows manufacturers to demonstrate that their new devices are as safe and effective as existing devices, known as predicate devices, thus avoiding the more extensive Pre-Market Approval process. To navigate this process successfully, manufacturers must thoroughly educate themselves on their device's intended use, potential users, and the competitive landscape.
This involves analyzing research literature, clinical studies, and existing products to craft a comparative assessment against potential predicate devices. The FDA's 510(k) database serves as a cornerstone for manufacturers, providing comprehensive information on cleared devices, helping to identify predicate devices, and offering insights for benchmarking and market analysis. Accessing this database is crucial for healthcare professionals, manufacturers, and researchers alike.
By following a step-by-step guide and utilizing advanced search features, users can effectively navigate the database and find the information they need. Understanding and interpreting search results is key to making informed decisions and ensuring device safety and efficacy. The database provides key information such as device characteristics, predicate devices, regulatory status, and additional details like recalls and warnings.
By leveraging the 510(k) database effectively, manufacturers can streamline the approval process and contribute to safer and more effective medical device innovations.
Understanding the 510(k) Clearance Process
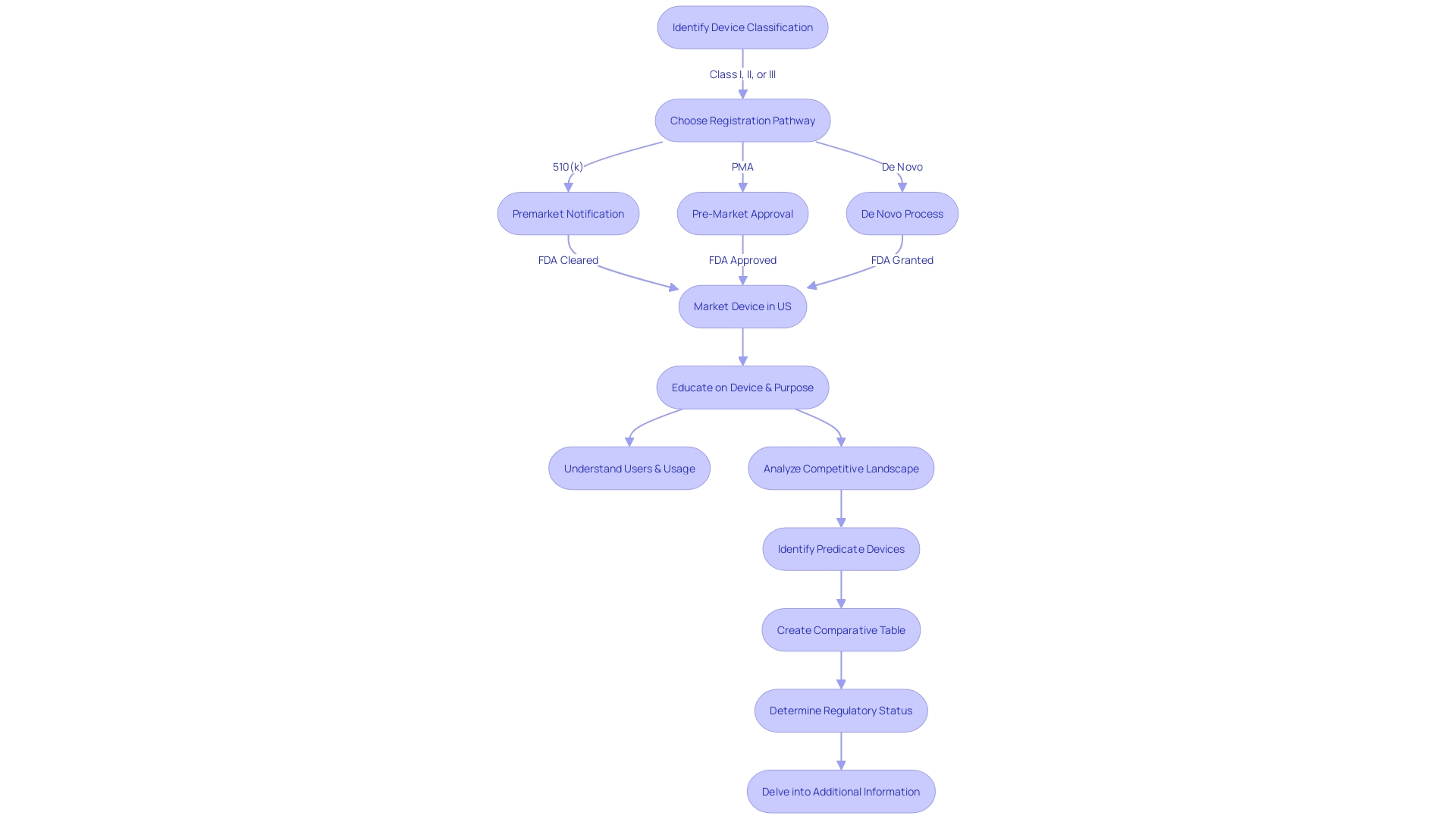
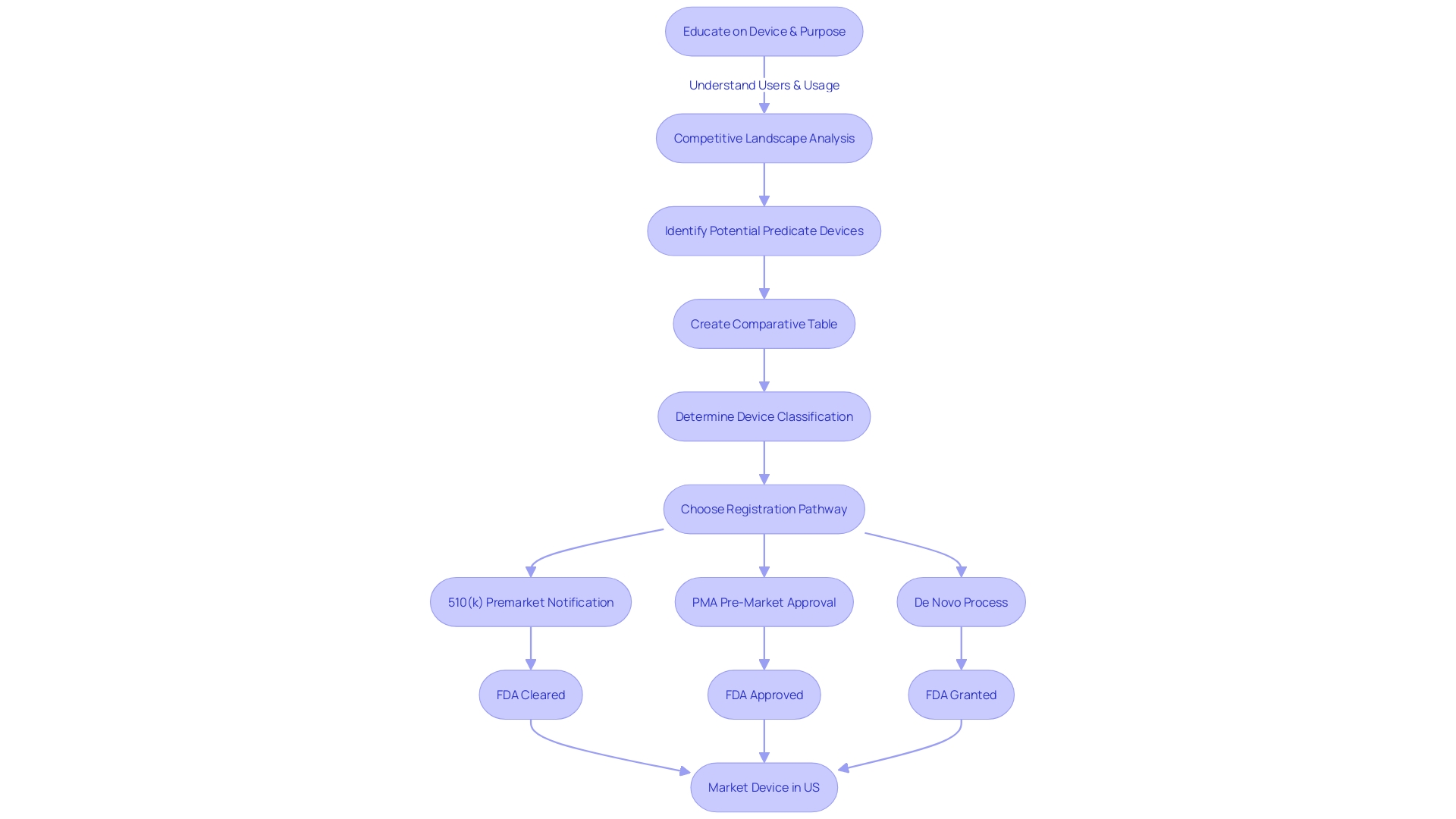
The 510(k) pathway, known as Premarket Notification, is an important element within the FDA's regulatory framework for medical instruments. It serves manufacturers looking to market products that are substantially equivalent to existing, legally marketed items, known as predicate devices. A successful 510(k) submission demonstrates that the new product is as safe and effective as the predicate, which allows the manufacturer to avoid the more extensive Pre-Market Approval (PMA) process. The FDA categorizes medical equipment into three groups based on the level of risk they pose to patients, with each group requiring a different regulatory approach. To navigate this process, manufacturers must rigorously educate themselves on the intended use of their product, potential users, and the existing competitive landscape. This requires a comprehensive examination of research literature, clinical studies, and existing products to create a comparative evaluation against potential reference products. These steps are crucial for aligning with FDA standards that emphasize consumer protection through assurance of instrument safety, effectiveness, and security. As the FDA continues to update its policies, such as the recent final rule on direct-to-consumer prescription drug advertisements, it underscores the importance of clear and accessible communication regarding healthcare products. Producers must stay knowledgeable and in accordance with these developing criteria to effectively introduce their healthcare equipment to the market.
Benefits of Using the 510(k) Database
The 510(k) serves as a cornerstone for manufacturers of medical equipment, providing a wealth of information that aids in the development, regulatory approval, and market positioning of new medical devices. It offers detailed data on approved equipment, including their intended purposes, indications, and technical specifications, which are vital for manufacturers seeking to innovate or improve current products. Moreover, the information system is vital in the identification of analogous equipment, allowing manufacturers to find and examine apparatus akin to their own, thereby simplifying the regulatory process by clarifying the prerequisites necessary for their equipment's approval.
An essential aspect of utilizing the 510(k) data involves the evaluation of regulatory history. This includes examining recalls, warnings, and adverse events associated with medical products, thereby empowering manufacturers to evaluate the safety and efficacy of their items more effectively. Moreover, the database is a valuable asset for benchmarking and market analysis, providing data that can be leveraged to understand market dynamics and to position products strategically against competitors.
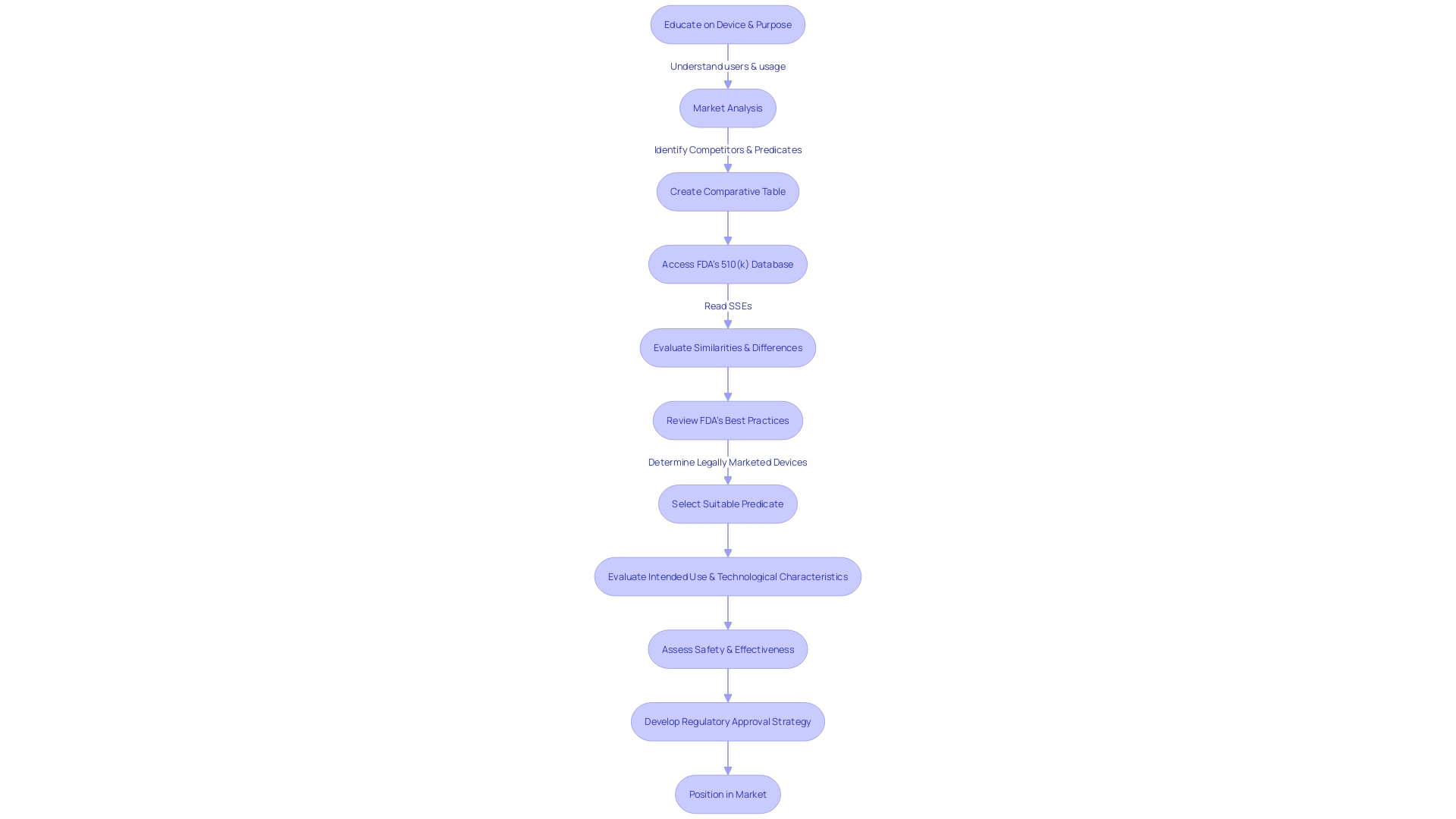
In light of the FDA's 510(k) modernization initiative, stakeholders now have access to draft guidance on best practices for selecting suitable predicates. This comes after a critical evaluation of the use of older predicates, recognizing the benefits such as the accumulation of long-term safety data. The first step in predicate selection involves confirming the legal market status of a potential predicate, followed by verifying its intended use and evaluating technological differences in terms of safety and effectiveness.
Highlighting the importance of a comprehensive understanding of the tool's users, including clinicians and patients, and its competitive environment is crucial. Manufacturers are recommended to carry out thorough research, utilizing sources such as literature, clinical studies, and marketing materials, to create comparative tables for previous models. This strategic approach is backed by the most recent technological advancements and market trends, guaranteeing that new healthcare equipment meets the changing demands of the industry while complying with regulatory standards.
How to Access the 510(k) Database
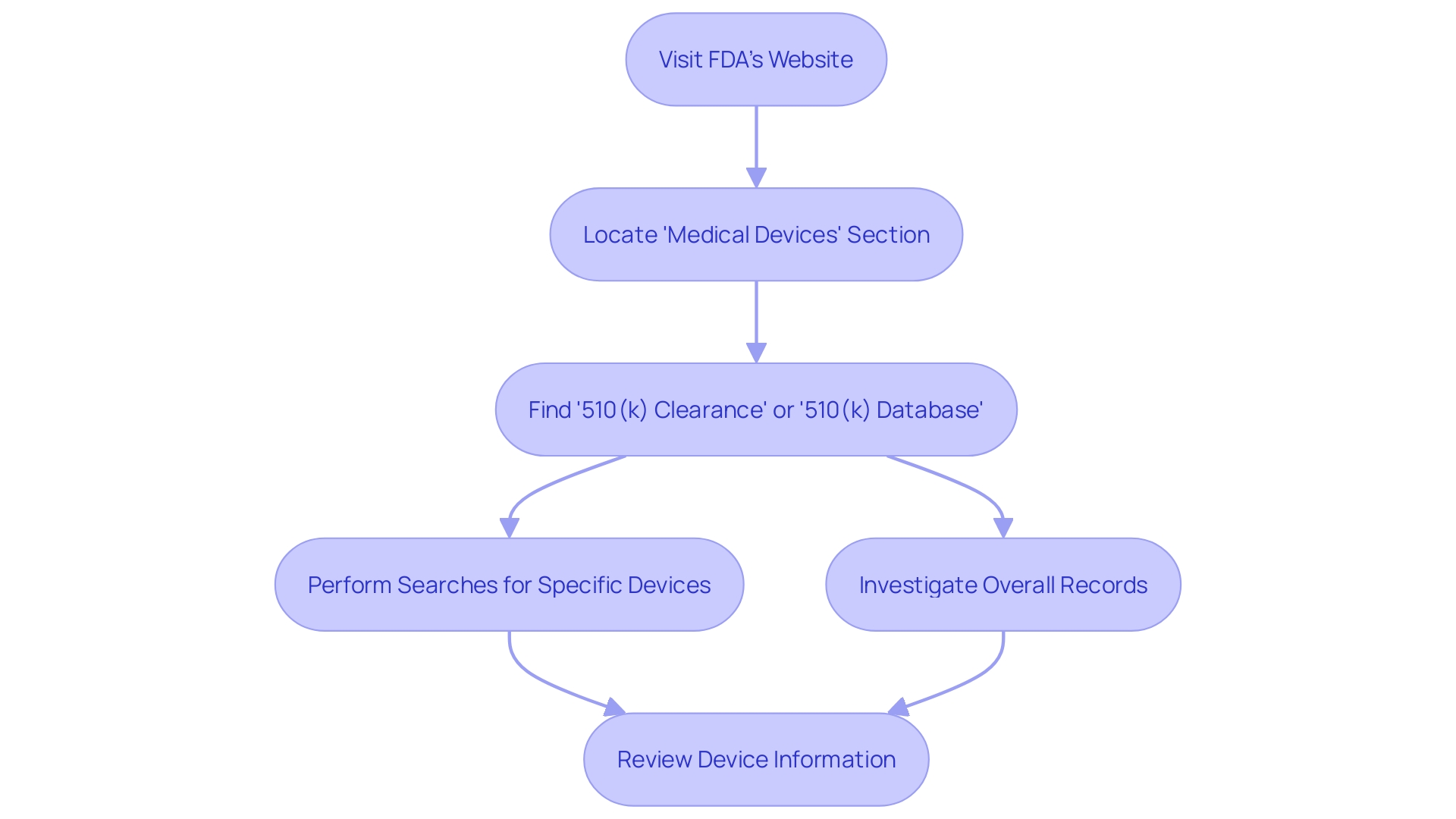
Exploring the 510(k) repository on the FDA's website enables a thorough comprehension of medical equipment, which is essential for healthcare professionals, manufacturers, and researchers alike. By following a few simple steps, individuals can access a wealth of information regarding gadgets that have been cleared for market. The process begins by visiting the FDA's website and locating the 'Medical Devices' section. Here, you will find the '510(k) Clearance' or '510(k) Database' option, which leads to a searchable collection of products.
Once in the data storage, users can effectively perform searches for particular gadgets or investigate overall records. It is crucial to acknowledge that the information system is not fixed; it is consistently updated as new gadgets receive authorization or when there are alterations to existing ones. Regular consultation of the information system is advised to stay abreast of the latest developments.
Furthermore, the information repository functions as a crucial asset for identifying predicate gadgets, which are indispensable for comparative assessments. Through the analysis of instruments with comparable intended purposes and technological attributes, it is possible to generate a comparative table—an invaluable resource delineated in a document entitled 'Unifying Safety and Velocity: An Approach Involving Humans and Algorithms to Augment the FDA's Policy for Clearing Medical Instruments.' This process is not only beneficial for understanding the competitive landscape but also for ensuring user safety, as emphasized in a documentary titled 'The Bleeding Edge,' which highlighted the intricacies of the FDA's clearance process.
In summary, the 510(k) repository is a dynamic and vital resource for anyone engaged in the healthcare equipment sector or public health. Its regular updates and comprehensive records make it an indispensable tool for ensuring the safety and efficacy of healthcare instruments.
Step-by-Step Guide to 510(k) Database Search
Exploring the 510(k) database for medical equipment approval necessitates a thorough comprehension of the product in question and its market context. Start by fully engaging in the subject matter, directing your attention towards users like clinicians and patients, and carefully examining the instructions for use of the equipment, which includes warnings and cautions. With insights from marketing teams, establish a firm grasp of the competitive landscape, reviewing resources like research literature, clinical studies, and materials from competitors to pinpoint potential predicate devices with analogous intended uses and technology.
Next, improve your search efficiency by utilizing specific search criteria that mirror the unique features, indications, and intended uses of the equipment. Utilize filters such as class, product code, and clearance date to enhance your search. Include accurate keywords to enhance the specificity of your results, and systematically assess the search output, paying careful attention to details like indications, clearances, and predicate tools.
Once you have a list of similar products, explore their regulatory history by consulting the Summaries of Safety and Effectiveness available in the database. This comparison should extend to analyzing technological characteristics, which can be methodically organized into a comparative table to aid in the assessment of your equipment's market readiness and regulatory compliance.
In your research, consider utilizing public access resources such as the SEC's EDGAR system to uncover filings that may provide additional insights into the market and regulatory landscape. As you extract and synthesize relevant information, stay updated on industry trends and regulatory updates to strategically inform your development process. By following this organized method, you will be fully prepared to navigate the 510(k) repository and assist your medical equipment approval efforts.
Utilizing Advanced Search Features
Leveraging the advanced search functionalities on the FDA's website can significantly enhance the precision and relevance of your 510(k) database queries. By using different search filters, you can narrow down your exploration to specific classes, product codes, and clearance dates. The inclusion of Boolean operators such as AND, OR, and NOT refines your search, allowing for the combination or exclusion of keywords, thus honing in on more accurate results. Wildcard characters like asterisks () or question marks (?) serve to broaden your search parameters, catching variations of a keyword to ensure comprehensive coverage. For instance, searching for 'implant' will yield results that include 'implants,' 'implantation,' and 'implanted.' Moreover, arrangement choices enable you to arrange your inquiry results by factors such as clearance date, which is especially helpful when prioritizing items relevant to your investigation.
In the context of 510(k) submissions, it's crucial to have a profound comprehension of the apparatus in question, including its users, instructions for use, and any associated warnings. Working together with Marketing to evaluate the competitive environment and identify possible preceding equipment is also crucial. The FDA's modernization initiative aims to enhance the review process, and the selection of an appropriate predicate, which is legally marketed and shares the same intended use without raising safety concerns, is a key step. As evidenced by recent FDA actions, such as the final rule on direct-to-consumer prescription drug advertisements, clear and understandable communication is essential. These standards apply not only to consumer ads but also to the presentation of 510(k) documentation, emphasizing the importance of clarity and transparency in all aspects of the FDA's regulatory activities.
Interpreting Search Results
When exploring the 510(k) database, it's important to delve deeper into the data to fully grasp the implications of the search results for the approval process of your medical product. Consider the following:
- Device Characteristics: Investigate the specific technological attributes and clinical applications of similar devices. This evaluation should include a comparison of intended use and technological features to ensure they are akin to your gadget.
Identifying predicate tools is a crucial step. These benchmark tools constitute the foundation for establishing substantial equivalence. Analyze their regulatory path and performance data to establish practical expectations for the approval pathway of your own product.
-
Regulatory Status: Determine the regulatory standing of items that emerged in your search. Whether they are already cleared or pending review, this information is crucial in forecasting the potential approval timespan and stipulations for your equipment.
-
Additional Information: Delve into supplementary data like recall histories, reported adverse events, or any warnings issued. Insights gleaned from this information can be invaluable in assessing equipment safety and efficacy.
By carefully examining the 510(k) search results, you can gather crucial information that will guide strategic choices and streamline the path to obtaining market approval for your product. It's crucial to highlight that the healthcare equipment panorama is constantly changing, with artificial intelligence technologies gaining importance in fields like diagnostic imaging and disease progression prediction. The regulatory environment is adapting accordingly to ensure the safe integration of these innovative tools into clinical practice.
In light of recent regulatory standards set by the FDA, such as those for direct-to-consumer prescription drug advertisements, it's clear that clarity, transparency, and patient comprehension are paramount. This same principle applies to the medical equipment clearance process, where manufacturers must present detailed, consumer-friendly information.
Furthermore, comprehensive understanding of the equipment, comprising its users, usage instructions, and any linked cautions, is crucial. Working together with Marketing to gain insights into the competitive landscape, and identifying previous tools through extensive research, is a crucial element of the preparation process. Creating a comparative table and reviewing Summaries of Safety and Effectiveness data can offer deeper insights into the approval likelihood and necessary compliance measures.
Statistics from the FDA's records demonstrate the scale of injuries and deaths associated with medical devices over a decade, emphasizing the importance of rigorous postmarket surveillance and proactive safety monitoring. As the industry leader Medtronic exemplifies, a commitment to innovative solutions and patient-centric care is key to addressing the complex health challenges of our time. A thorough approach to the 510(k) clearance process is in line with this objective, guaranteeing that new healthcare tools are not only efficient but also secure for the end-users.
Key Information Available in the 510(k) Database
The 510(k) information repository is an invaluable resource for manufacturers and stakeholders in the healthcare equipment industry, providing access to vital data for ensuring regulatory compliance and facilitating the approval process. By utilizing this data repository, one can analyze comprehensive aspects of devices, encompassing their structure, operational features, and intended purpose. For example, understanding the exact indications for the utilization of a tool is essential, as it specifies the medical conditions or purposes the tool targets.
The database also highlights predicate instruments, which are crucial for manufacturers seeking to demonstrate substantial equivalence to previously cleared devices. Evaluating the regulatory history is another important aspect, offering insights into recalls, warnings, or adverse events that may affect the safety and effectiveness of a product. Clearance information, such as the date and regulation under which an item was cleared, is equally important for maintaining up-to-date knowledge of an item's status.
This data becomes especially crucial in view of the over 1.7 million injuries and 83,000 deaths potentially associated with healthcare equipment during a decade in the U.S., highlighting the significance of thorough postmarket monitoring and safety evaluations. The FDA has been actively working to establish a strong postmarket surveillance system, acknowledging the need for ongoing vigilance in the healthcare instrument industry.
The information gleaned from the 510(k) database is not only a regulatory requirement but also serves as a knowledge base for companies like Medtronic, a leading healthcare technology company that leverages such data to deliver life-changing technologies and therapies. With a goal to relieve pain, improve health, and prolong life, Medtronic's dedication to innovation is apparent in its wide range of medical instruments that tackle a variety of health conditions.
To effectively navigate the 510(k) process, manufacturers must undertake a comprehensive understanding of the product in question, its users, and the competitive landscape. This involves examining research literature, clinical studies, and competitive marketing materials to identify appropriate reference devices. To identify the resemblances and distinctions between devices, a comparative examination is crucial, as indicated by the Summaries of Safety and Effectiveness accessible on the FDA's platform.
The FDA's guidance documents offer a roadmap for planning and executing a 510(k) submission, with the goal of demonstrating substantial equivalence. The challenge lies in meticulously compiling the necessary information within the allotted time frame, ensuring it aligns with the FDA's expectations. As the FDA continues to modernize the 510(k) process, it has released draft guidance on selecting predicates, emphasizing the importance of choosing legally marketed products with established safety and effectiveness.
In general, the strategic utilization of the 510(k) record can empower producers to make knowledgeable choices, streamline the approval process, and ultimately contribute to safer and more effective innovations for patients.
Tips for Effective 510(k) Database Search
Becoming proficient in the 510(k) medical approval process is essential for ensuring that medical instruments meet safety and effectiveness standards. To maximize the potential of the 510(k) database, begin by thoroughly exploring the purpose and target audience of the equipment, including clinicians, patients, and any specific usage warnings. This knowledge lays the groundwork for a strategic search. Use specific keywords that match the class, product code, and critical attributes of the equipment to effectively filter search results. Don't limit your research to a singular tool; analyzing a variety of instruments expands your viewpoint on industry standards and expectations. Comparing your equipment against predicate objects is crucial, as it provides insights into substantial equivalence and the nuances of regulatory requirements. Stay updated on the latest developments by regularly visiting the 510(k) data repository, ensuring access to the most up-to-date information. These steps will not only optimize your 510(k) search but also strengthen your regulatory approach for medical product development.
Common Challenges and Solutions
When using the 510(k) system, users might encounter some obstacles, but there are strategies to effectively manage these challenges. If you're encountering limited search results, consider broadening your search terms or simplifying your filters for a wider array of results. For those dealing with the complicated categorization of gadgets, it is advantageous to refer to FDA's classification guidance or consult regulatory experts for a clearer comprehension. If the information in the system is outdated, it is wise to cross-check the data with other credible sources or directly communicate with the FDA for the latest updates.
To successfully navigate the 510(k) database, one needs a thorough comprehension of the subject product, including its intended users and detailed instructions for use. Marketing insights can also assist in evaluating the competitive landscape, enabling a thorough comparison of similar products. A valuable resource in this process is the FDA's Summaries of Safety and Effectiveness, which can be instrumental in evaluating similarities and differences between instruments.
Moreover, the FDA's ongoing efforts to modernize the 510(k) process include developing best practices for selecting predicates, as outlined in their recent draft guidance. Ensuring that a potential predicate is legally marketed and shares the same intended use without raising new safety concerns is crucial in this selection.
Given the increasing dietary supplement market, the National Institutes of Health's Dietary Supplement Label Database (DSLD) serves as an illustration of a sturdy repository that captures extensive product information to assist users in staying informed about the ever-evolving landscape of supplements.
It's important to stay informed about recent news and updates from the FDA and the industry to maintain a keen understanding of regulatory environments and safety issues related to medical equipment. By remaining vigilant and utilizing available resources and expert guidance, users can successfully navigate the complexities of the 510(k) database and make informed decisions in the medical device field.
Conclusion
In conclusion, the 510(k) clearance process is a critical step for medical device manufacturers. Thorough education on the device's intended use and the competitive landscape is essential for successful navigation. The FDA's 510(k) database serves as a cornerstone, providing comprehensive information on cleared devices and helping to identify predicate devices.
Access to this database is crucial for healthcare professionals, manufacturers, and researchers.
Utilizing advanced search features and following a step-by-step guide allows users to effectively find the information they need in the 510(k) database. Understanding and interpreting search results is key to making informed decisions and ensuring device safety and efficacy.
The database offers key information such as device characteristics, predicate devices, regulatory status, and additional details like recalls and warnings. By leveraging the database effectively, manufacturers can streamline the approval process and contribute to safer and more effective medical device innovations.
Interpreting search results involves investigating device characteristics, identifying predicate devices, ascertaining regulatory status, and delving into additional information. Meticulous analysis informs strategic decisions and streamlines the device approval process.
To effectively navigate the 510(k) database, manufacturers must undertake a comprehensive understanding of the device, its users, and the competitive landscape. Utilizing precise keywords, comparing devices against predicate devices, and regularly visiting the database are essential steps. These strategies empower manufacturers to make informed decisions, streamline the approval process, and contribute to safer and more effective medical device innovations.
While challenges may arise when utilizing the 510(k) database, solutions exist to effectively manage them. Broadening search terms, simplifying filters, referring to FDA guidance, and verifying data against reliable sources are strategies to overcome obstacles. Deep understanding of the subject device, collaboration with marketing, and utilization of the FDA's Summaries of Safety and Effectiveness aid in navigating the database.
Staying informed about recent news and updates from the FDA and the industry is crucial for understanding regulatory environments and device safety issues. By remaining vigilant and utilizing available resources and expert guidance, users successfully navigate the complexities of the 510(k) database and make informed decisions in the medical device field.




