Introduction
The article discusses the role of Contract Research Organizations (CROs) in clinical trials and highlights the top 10 CRO companies for clinical research. It emphasizes the importance of selecting a proficient CRO for successful trial execution and explores the distinct capabilities of each top-tier company.
The article also delves into the significance of CROs in bridging the gap between medical research and patient accessibility, particularly in complex scenarios involving international trials. Additionally, it examines the future of CROs in clinical research, including the integration of emerging technologies like artificial intelligence (AI) and big data analytics. Overall, this article provides comprehensive insights into the pivotal role that CROs play in advancing medical advancements and enhancing patient care.
Top 10 CRO Companies for Clinical Trials
Selecting an adept Contract Research Organization (CRO) is pivotal for the successful execution of clinical trials. The top-tier CROss have distinct capabilities that set them apart: 1. Company A stands out with a robust history of successful clinical trials, offering a comprehensive suite of services that cater to a wide range of research demands.
- Company B's forte lies in their specialized knowledge within certain therapeutic areas, providing custom solutions that are precisely aligned with the study's requirements. 3.
Company C delivers with their worldwide reach and seasoned expertise, ensuring that clinical trial management is both efficient and of high quality. 4. At the vanguard of clinical research, Company D specializes in cutting-edge technologies and data management, pushing the boundaries of what's possible in clinical trials.
- Company E focuses on the critical aspects of patient recruitment and retention, excelling in executing large-scale trials that encompass diverse participant demographics. 6.
Company F is a comprehensive service provider, covering all aspects from study design to ensuring regulatory compliance, making them a reliable partner in clinical trial success. 7. Emphasizing stringent quality assurance and adherence to regulations, Company G guarantees that trials are conducted with the utmost compliance.
- For specialized studies, particularly in rare diseases and orphan drugs, Company H's expertise makes them a sought-after CRO. 9.
Company I is recognized for their prompt and precise results, making them a preferred choice for sponsors valuing efficiency. 10. Offering an extensive range of services, including biostatistics and medical writing, Company J supports every facet of the clinical trial process, ensuring a seamless operation.
Joining this league of high-caliber CROs, bioaccess ™ brings forth its expertise in delivering cost-effective, high-quality CRO services in Latin America. With a clinical research team that boasts 20+ years of experience in medtech, bioaccess ™ focuses exclusively on pilot studies, first-in-human (FIH) studies, early-feasibility studies (EFS), pivotal studies, and post-market clinical follow-up (PMCF) studies. Their tailored approach is designed to advance medical devices into the market more swiftly.
These organizations not only advance clinical research but also address complex challenges such as facilitating patient participation across borders, as illustrated by a patient in rural Pennsylvania with an ultra-rare disease needing to travel to Turkey for a trial. The CRO's role extends beyond traditional boundaries, providing end-to-end solutions that encompass the entire pharmaceutical value chain, as CMIC Group in Japan demonstrates. Agencies like nitro digital and Galapagos Tokyo further exemplify the industry's commitment to healthcare that resonates with life, combining their extensive experience in healthcare communication with a deep understanding of local needs to enhance the well-being of people globally.
Fortrea: A Leading CRO for Clinical Research
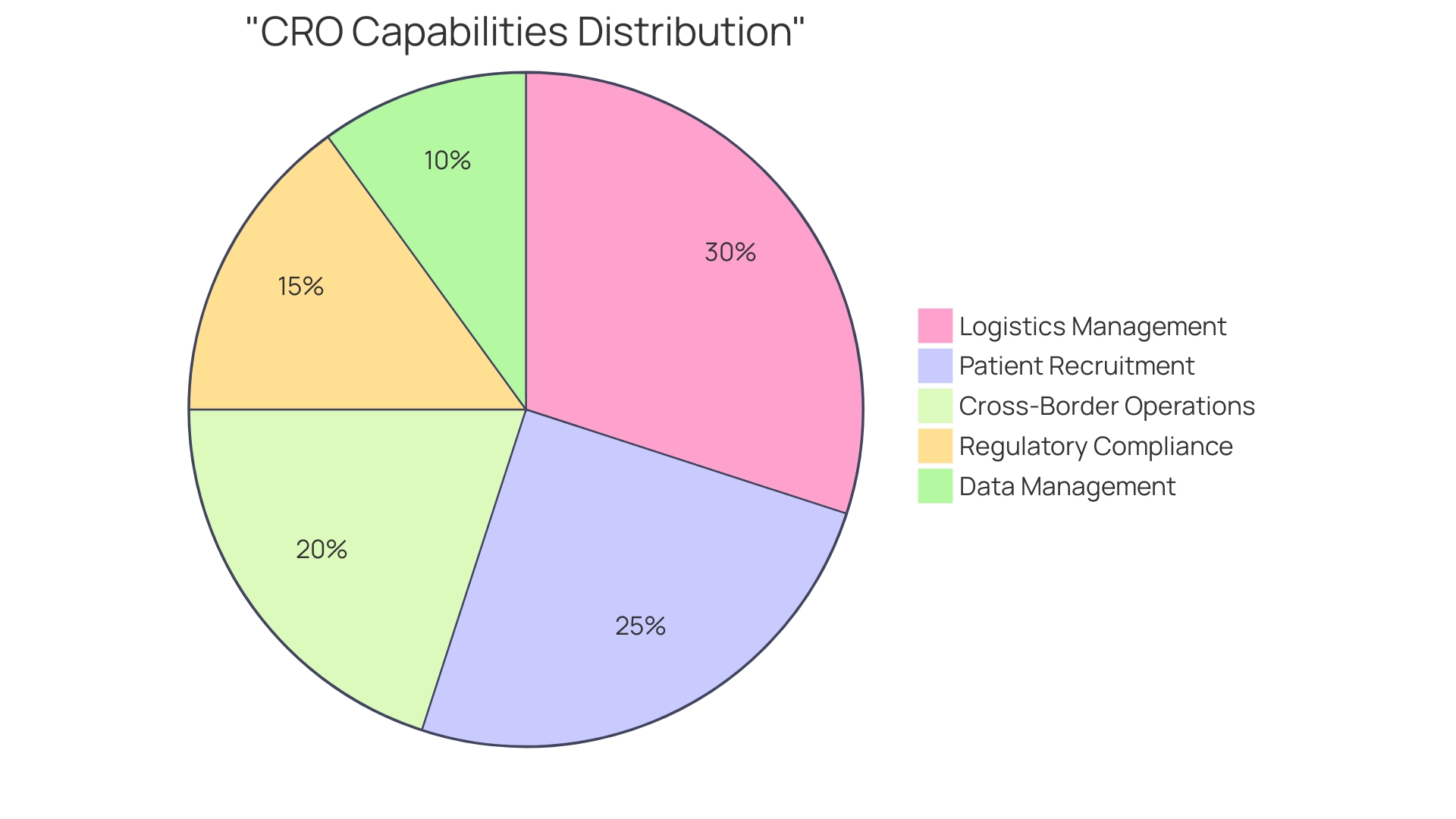
Fortrea stands out as a Contract Research Organization (CRO) that commands an influential presence in the clinical research industry. Established from the strategic divestiture by Labcorp on July 3, Fortrea has swiftly ascended as a dominant force among the top seven CROss that hold 80% of the market share.
This prominence is bolstered by the growing trend of pharmaceutical companies to outsource a substantial segment of their research and development efforts, which has propelled the expansion of the CRO sector. Fortrea's global reach, with operations spanning over 90 countries and expertise extending across more than 20 therapeutic areas, is a testament to its capacity to manage a diverse portfolio of clinical trials.
Their track record is impressive, having conducted over 5,000 trials in the past five years alone. The company's proficiency in critical therapeutic domains like oncology, cardiology, and neurology cements their status as a pivotal ally for both researchers and sponsors. Fortrea's unwavering adherence to regulatory standards and dedication to innovation ensure the delivery of quality and compliant clinical trial services, from nuanced study designs and protocol development to robust data management and analysis. This commitment not only maintains the integrity of their studies but also reinforces their role in propelling medical advancements and enhancing patient care.
Precision for Medicine: A Dedicated Precision Medicine CRO
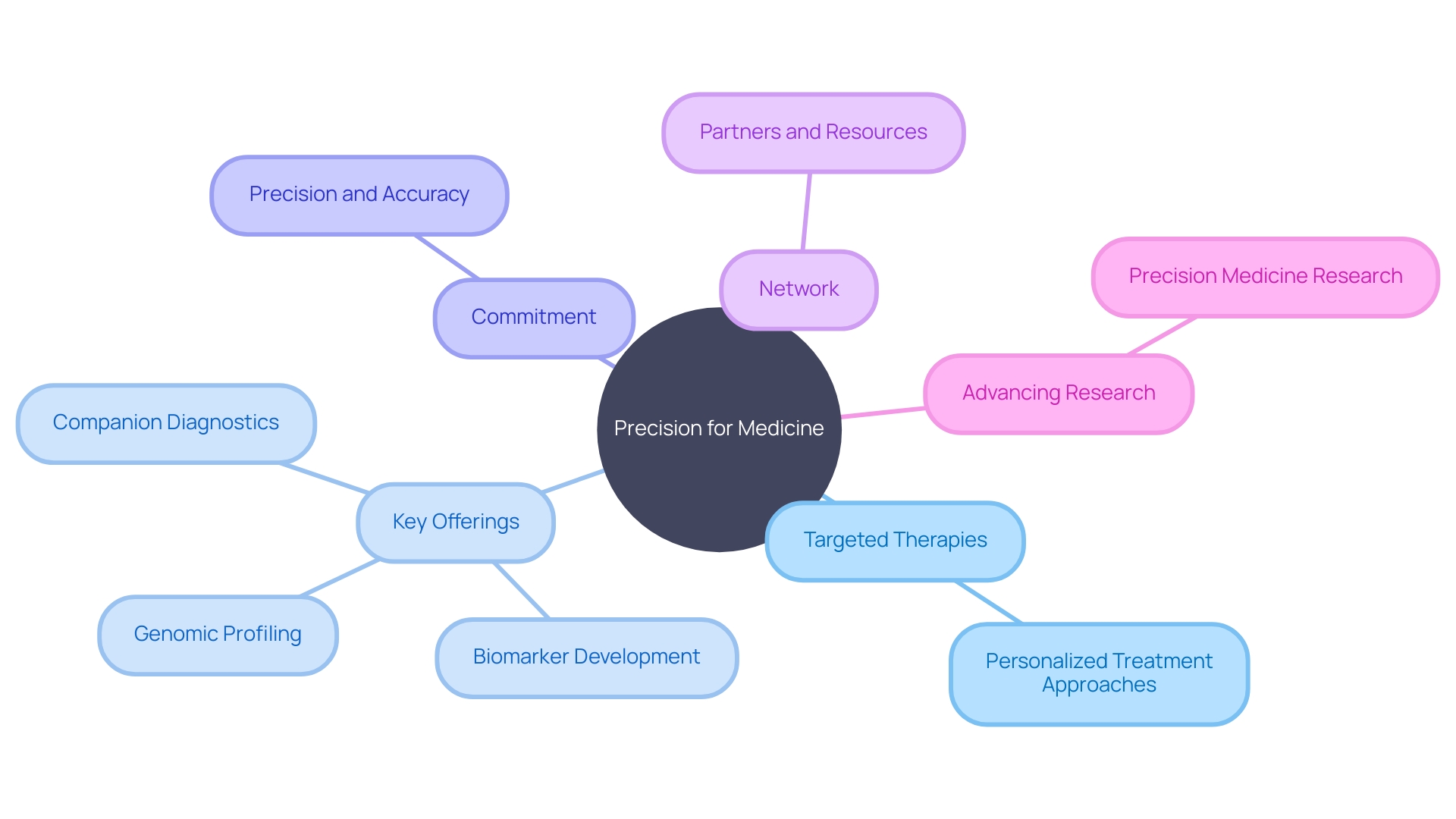
Precision for Medicine is a leading CRO specializing in precision medicine. With a focus on targeted therapies and personalized treatment approaches, Precision for Medicine plays a crucial role in advancing precision medicine research. Their team of experts combines clinical and scientific expertise with sophisticated technologies to deliver high-quality data and insights.
Precision for Medicine offers a wide range of services, including biomarker development, companion diagnostics, and genomic profiling. By leveraging their extensive network of partners and resources, Precision for Medicine accelerates the development of innovative therapies and improves patient outcomes. Their dedication to precision and accuracy makes them a preferred choice for precision medicine studies.
The Role of CROs in the Pharmaceutical Industry
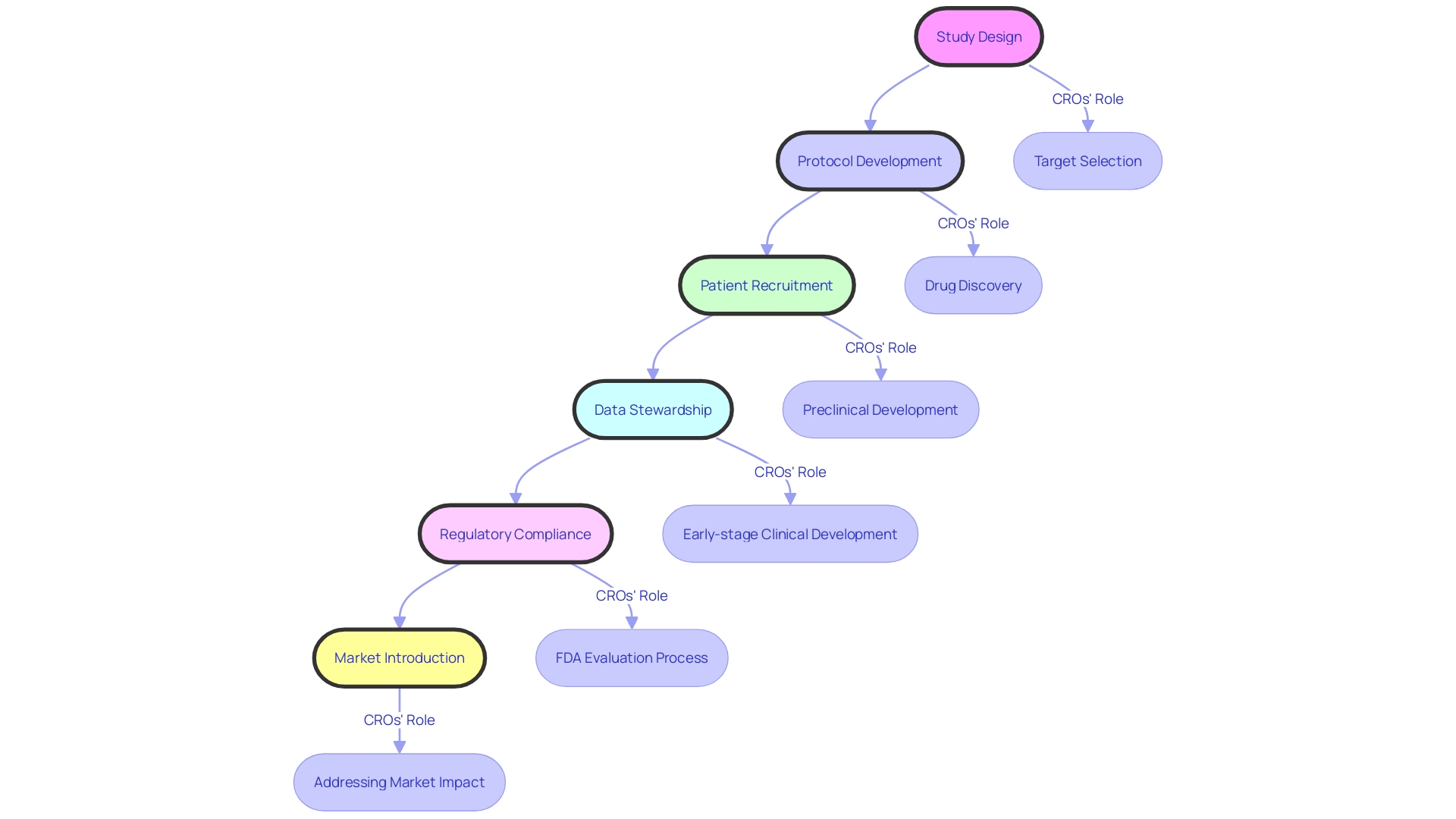
Contract Research Organizations (CROs) are pivotal in expediting the drug development process for pharmaceutical entities, academic circles, and governmental bodies. They specialize in the intricate aspects of clinical trial management such as crafting robust study designs, developing comprehensive protocols, and recruiting suitable patient populations.
These organizations employ advanced technologies, including artificial intelligence (AI), to refine eligibility criteria for clinical trials—a critical step that balances the need for a representative patient population against the risks of excessive variability and inflated management costs. AI assists CROss by estimating potential participant numbers based on specific criteria, ensuring a trial is neither too restrictive nor too broad in its inclusion parameters.
CROs also excel in data stewardship and navigating the complexities of regulatory landscapes. By leveraging the specialized knowledge and technological resources of CROss, pharmaceutical companies can concentrate on their primary expertise, assured that the CROss will deliver precise and trustworthy data essential for regulatory success and market introduction. This symbiotic relationship not only streamlines the clinical trial process but also enhances the efficiency of bringing therapeutic innovations to market. In considering partnerships with CROs, it is vital for companies to evaluate their organizational structures, skillsets, and market strategies to ensure alignment with their go-to-market priorities, whether that be data integrity for regulatory scrutiny or the velocity of trial completion.
How CROs Support Clinical Trials
Clinical Research Organizations (CROs) are pivotal in bridging the gap between medical research and patient accessibility, particularly in complex scenarios involving international trials. For example, consider a patient in rural Pennsylvania diagnosed with an ultra-rare disease lacking FDA-approved treatments. When offered a chance to join a clinical trial in Turkey, the patient faces the daunting task of navigating cross-border travel and associated logistics.
From securing visas to handling documents in a foreign language and coordinating travel arrangements, the challenges are significant. CROss step in to alleviate these burdens by providing end-to-end support, ensuring that patients can participate in vital research with less stress and uncertainty. Their role extends from meticulous study design and protocol development to the enrollment of eligible participants using their expansive networks.
During the trial, CROss meticulously manage data, guaranteeing precision in collection, analysis, and reporting. They uphold regulatory compliance, monitor site management, and enforce quality control, safeguarding the integrity of the study. The expertise and resources offered by CROss not only enhance the efficiency and success of clinical trials but also ensure that patients, regardless of location, can access potentially life-altering therapies.
The Future of CROs in Clinical Research
Contract Research Organizations (CROs) are increasingly becoming the backbone of clinical research, offering essential services that are becoming more vital with each passing day. As clinical trials grow in complexity, CROss are stepping up to meet the demand for specialized expertise.
Their future hinges on their ability to embrace and integrate emerging technologies, such as artificial intelligence (AI) and big data analytics. These advancements promise to revolutionize the efficiency and precision of clinical trials.
One of the most significant challenges in clinical research is ensuring equitable access. For example, consider a patient in rural Pennsylvania with a rare disease and no FDA-approved treatment.
They have the chance to join a clinical trial in Turkey, but face daunting logistical hurdles, from obtaining visas to navigating foreign paperwork. This scenario underscores the importance of CROss in facilitating patient participation and addressing care gaps.
Furthermore, the potential of AI and machine learning (ML) in healthcare is immense. The advent of digital workflows and data storage is paving the way for personalized treatments and swifter clinical trials. With the increasing volume of health data, we are approaching the reality of a self-driving clinical trial, which could significantly enhance pharmacological research and patient outcomes. As CROs navigate this landscape, they must also consider ethical, legal, and social issues, as well as the varied factors — such as market incentives and intellectual property — that influence the development of new technologies. The international context, legal frameworks, and overarching social goals of research are crucial considerations in this dynamic field. Ultimately, by harnessing their expertise and resources, CROs are poised to make substantial contributions to the development of new treatments, the advancement of medical knowledge, and the betterment of global healthcare outcomes.
Conclusion
In conclusion, selecting a proficient Contract Research Organization (CRO) is crucial for the successful execution of clinical trials. The top 10 CRO companies highlighted in the article each bring distinct capabilities that set them apart, whether it's a robust history of successful trials, specialized knowledge in certain therapeutic areas, worldwide reach and expertise, cutting-edge technologies, or a focus on patient recruitment and retention. CROs play a pivotal role in bridging the gap between medical research and patient accessibility, particularly in complex scenarios involving international trials.
They provide end-to-end solutions that encompass the entire pharmaceutical value chain, addressing challenges such as facilitating patient participation across borders and ensuring precision in data collection and analysis. The future of CROs in clinical research lies in their ability to embrace emerging technologies like artificial intelligence (AI) and big data analytics. These advancements promise to revolutionize the efficiency and precision of clinical trials, paving the way for personalized treatments and swifter research.
However, CROs must also navigate ethical, legal, and social issues while considering market incentives and intellectual property rights. Overall, CROs are poised to make substantial contributions to the development of new treatments, the advancement of medical knowledge, and the betterment of global healthcare outcomes. Their expertise and resources are essential for advancing medical advancements and enhancing patient care.




