Introduction
Contract Research Organizations (CROs) play a vital role in the field of medical research by providing essential services for clinical trials. From regulatory support to data management and medical writing, CROs navigate the complexities of clinical trials.
This article explores various types of CROs and their specialized expertise in different areas, such as full-service CROs, niche CROs, virtual CROs, regional CROs, and academic CROs. Each type offers unique solutions to overcome challenges in clinical research, ensuring the advancement of public health and the well-being of patients.
Types of CROs
Contract Research Organizations (CROs) have become indispensable in the realm of medical research by offering an array of services crucial for the progression of clinical trials. These organizations are adept at navigating the complexities of clinical trials, providing indispensable regulatory support, data management, monitoring, and medical writing services.
A poignant case that sheds light on the critical role of CROss involves a patient from rural Pennsylvania with an ultra-rare disease. Faced with no FDA-approved treatment options, they are presented with the chance to partake in a clinical trial in Turkey.
Here, the logistical challenges of international travel come into play, raising questions about visas, language barriers, and travel coordination. This scenario underscores the importance of CROss in facilitating such cross-border clinical research endeavors.
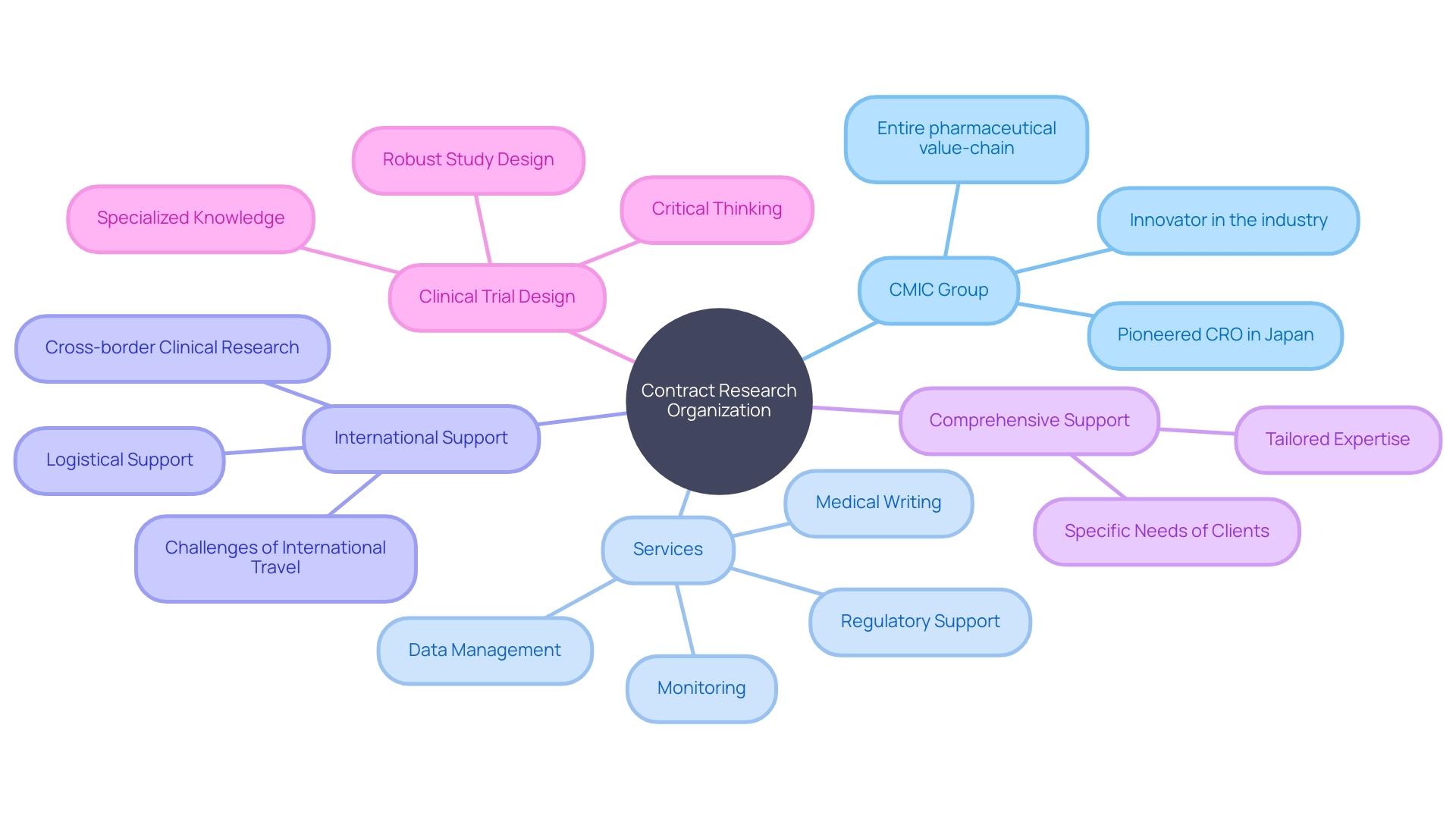
CMIC Group, Japan's pioneering CRO, exemplifies the comprehensive support that CROss can offer, extending services throughout the pharmaceutical value chain. From development and manufacturing to market entry solutions, CROss like CMIC tailor their expertise to meet the specific needs of their clients, whether they are pharmaceutical companies, medical device manufacturers, or academic institutions. Highlighting the importance of robust study design, an epidemiologist emphasizes the critical thinking required to prevent and address potential issues in clinical trials, such as bias and confounding factors. These insights further illustrate the specialized knowledge and expertise that CROss bring to the table, ensuring that studies are meticulously designed to answer pivotal questions and ultimately advance public health.
Full-Service CROs
Engaging a full-service Contract Research Organization (CRO) can be a pivotal decision for patients facing the complexity of participating in clinical trials abroad. For instance, a patient from rural Pennsylvania grappling with an ultra-rare disease for which there are no approved treatments may be presented with the chance to join a trial in Turkey.
However, the logistical challenges of international travel pose significant hurdles. A full-service CRO offers comprehensive solutions, addressing everything from study design to regulatory submissions, while also managing the intricate details of site selection, patient recruitment, and project oversight. Crucially, they can streamline processes that might otherwise overwhelm a patient, such as obtaining travel visas, navigating foreign paperwork, and coordinating travel arrangements, thereby alleviating the stress of logistics and allowing the patient to focus on their health and treatment.
Niche CROs
Navigating the complexities of clinical trials, particularly those with specific therapeutic focuses, calls for a tailored approach that niche Contract Research Organizations (CROss) are exceptionally equipped to provide. These CROss harbor a wealth of specialized knowledge, from oncology to cardiology, and are adept at managing the intricacies of early-phase studies. Their expertise is not just in the science but also in patient-centric practices that prioritize the participant's experience throughout the trial.
By ensuring that patients' perspectives and needs are integral to the trial design, niche CROs can address the diverse challenges patients face, such as language barriers and logistics of international travel, as highlighted by the scenario of a patient from rural Pennsylvania traveling to Turkey for a trial. This approach aligns with industry expert Daniel J Herron's emphasis on patient-centricity, which includes making trial information accessible and understandable, thereby reinforcing the importance of diversity, equity, and inclusion in clinical research. Niche CROs are not only equipped to implement hypothesis-driven trials with detailed planning and contingency strategies, but they also ensure adherence to protocols and regulatory practices such as GCP, GLP, and GMP, which are critical for the success and ethical integrity of the trial.
Virtual CROs
The integration of artificial intelligence (AI) and machine learning (ML) in the healthcare sector is transforming the landscape of clinical trials. Virtual Contract Research Organizations (CROs), or eCROs, harness these advanced technologies to facilitate trials with unprecedented efficiency and precision.
By employing digital workflows and data management systems, eCROs are not bound by the confines of physical infrastructure. Rather, they operate through a network of remote experts and strategic partnerships, which grants them the ability to scale operations to suit various project sizes, including those with limited resources.
This model is particularly beneficial for patients with rare diseases who may otherwise face barriers to participation in clinical trials due to geographical and logistical challenges. Imagine a patient in rural Pennsylvania, suffering from an ultra-rare condition, being offered a chance to partake in a clinical trial based in Turkey. The eCRO model streamlines their involvement by mitigating the complexities of international travel and language barriers, thus expanding access to potentially lifesaving treatments. As health data proliferates, the concept of a self-driving clinical trial is becoming more tangible, promising to revolutionize patient care and enhance the outcomes of pharmacological research.
Regional CROs
Regional Contract Research Organizations (CROs) serve as specialized partners for clinical studies, particularly within their own geographic domains. Their expertise extends to a granular understanding of local regulatory requirements, such as the intricate details of the US Food and Drug Administration Form 1572 (21 CFR 312.53[c]), which delineates the infrastructure necessary for patient participation at a local level. This includes the identification of medical institutions, hospitals, and research facilities where clinical investigations are conducted, as well as the clinical laboratory facilities utilized for the study.
With their finger on the pulse of healthcare infrastructure and cultural contexts, regional CROss offer invaluable insights and operational capabilities, ensuring compliance and data integrity. This is akin to the approach needed in the home-based care sector, as highlighted by Luke Rutledge, Chief Commercial Officer at Homecare Homebase, who emphasizes the importance of a nuanced understanding of the sector's challenges and opportunities to meet the booming demand for patient-centric solutions. Regional CROss mirror this philosophy by providing tailored support that aligns with the unique requirements of each study location, thereby streamlining the pathway for researchers to generate robust and compliant clinical data.
Academic CROs
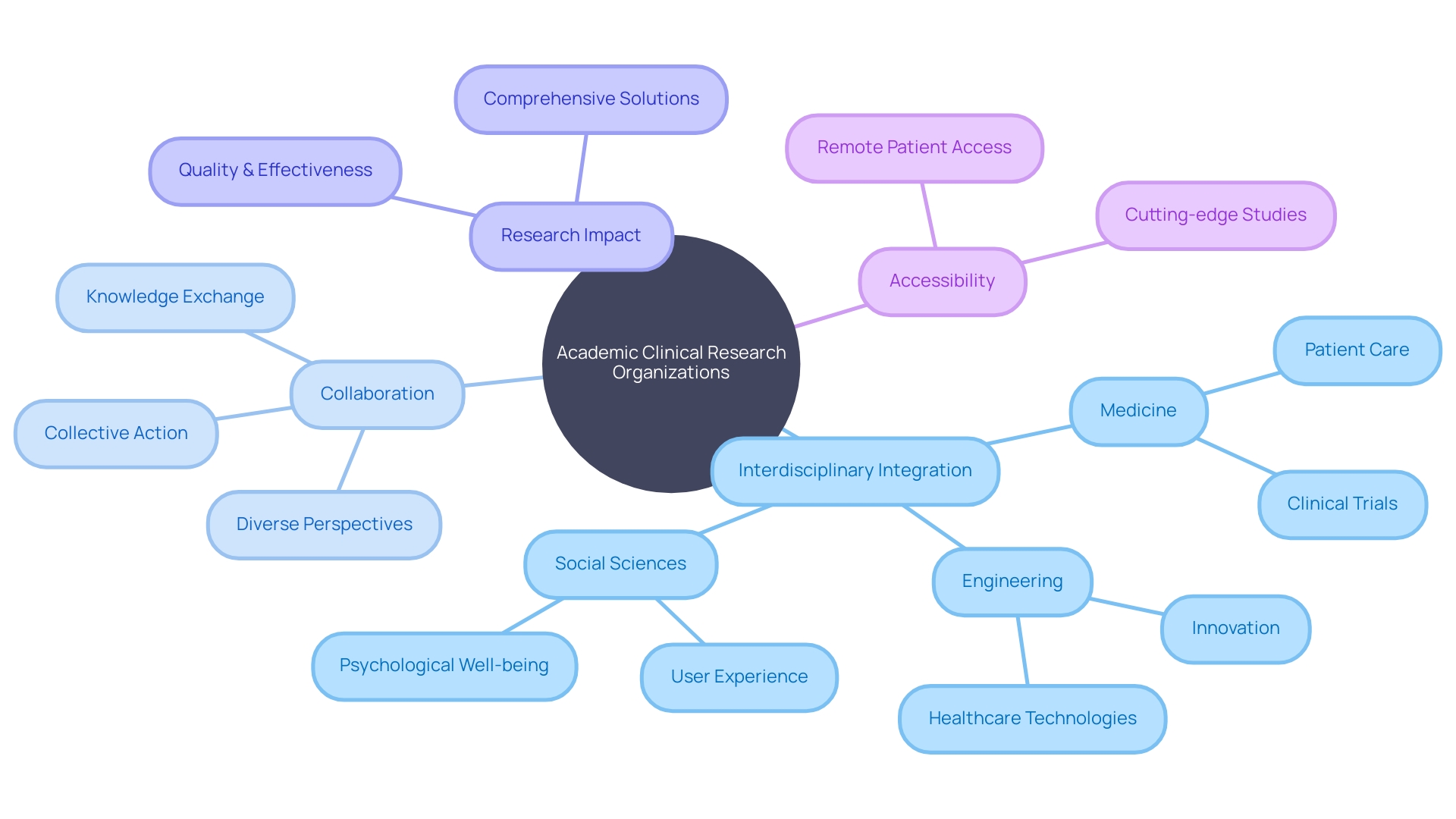
Academic Clinical Research Organizations (CROs), with their foundation in universities and academic settings, offer a unique convergence of scholarly insight and clinical acumen. These organizations are adept at orchestrating clinical trials and research studies through the synergy of academic intellectuals and researchers. Key to their success is the seamless access to specialized infrastructure, a broad spectrum of patient demographics, and in-depth scientific knowledge—assets that position them as indispensable collaborators for industry-funded and independent research investigations.
A compelling example of the power of collaboration within these entities is the interdisciplinary integration of medicine, engineering, and social sciences, which can spawn groundbreaking healthcare innovations that encapsulate medical efficacy, user-centric design, and psychological considerations. This amalgamation of expertise not only propels scientific discovery but also elevates the practicality and impact of research outcomes. Moreover, the collaborative spirit that academic CROss embody is pivotal to cultivating new insights and advancing healthcare paradigms, ensuring that even patients in the most remote locations have the potential to benefit from cutting-edge clinical trials and studies.
Conclusion
In conclusion, Contract Research Organizations (CROs) play a vital role in medical research by providing essential services for clinical trials. Full-service CROs streamline participation in trials abroad, addressing logistical challenges and allowing patients to focus on their health.
Niche CROs specialize in specific therapeutic areas, prioritizing patient-centric practices and ensuring trial success. Virtual CROs leverage advanced technologies to facilitate trials efficiently, expanding access for patients with rare diseases.
Regional CROs offer local expertise, ensuring compliance and data integrity. Academic CROs foster interdisciplinary collaboration, driving innovative healthcare solutions. Together, these diverse types of CROs advance medical research and improve patient outcomes.




