Introduction
Navigating the FDA's 510(k) premarket notification terrain requires a comprehensive understanding of the FDA's classification system for medical devices. This system determines the most appropriate pathway to market based on the risk the devices pose to patients. Whether it's through the 510(k) Premarket Notification, Pre-Market Approval (PMA), or the De Novo process, devices must undergo specific submissions to enter the U.S. market.
Distinguishing between FDA Cleared, FDA Approved, and Granted devices is crucial, as they reflect different levels of regulatory scrutiny and approval. The 510(k) process plays a vital role in clearing medical devices that demonstrate substantial equivalence to a legally marketed device. Conducting thorough research, analyzing the competitive landscape, and consulting the Summaries of Safety and Effectiveness Data (SSEDs) in the FDA's 510(k) database are essential steps in this process.
Transparency in the submission process is also emphasized, as all comments and documents may become public. Navigating the FDA 510(k) Search is indispensable for regulatory professionals in the medical device industry, ensuring compliance and success in a highly regulated market.
What is the FDA 510(k) Search?
Navigating the FDA's 510(k) premarket notification terrain requires a comprehensive understanding of the FDA's classification system for medical devices, which is pivotal in determining the most appropriate pathway to market. The FDA classifies devices into three levels based on the risk they pose to patients, with each classification requiring a different type of submission—namely, 510(k) Premarket Notification, Pre-Market Approval (PMA), or the De Novo process.
For a device to enter the U.S. market, it must be FDA Cleared (for Class I or II devices through the 510(k) process), FDA Approved (for Class III devices through the PMA process), or Granted (for devices that successfully pass the De Novo process). It's essential to distinguish between these terms, as they reflect different levels of regulatory scrutiny and approval.
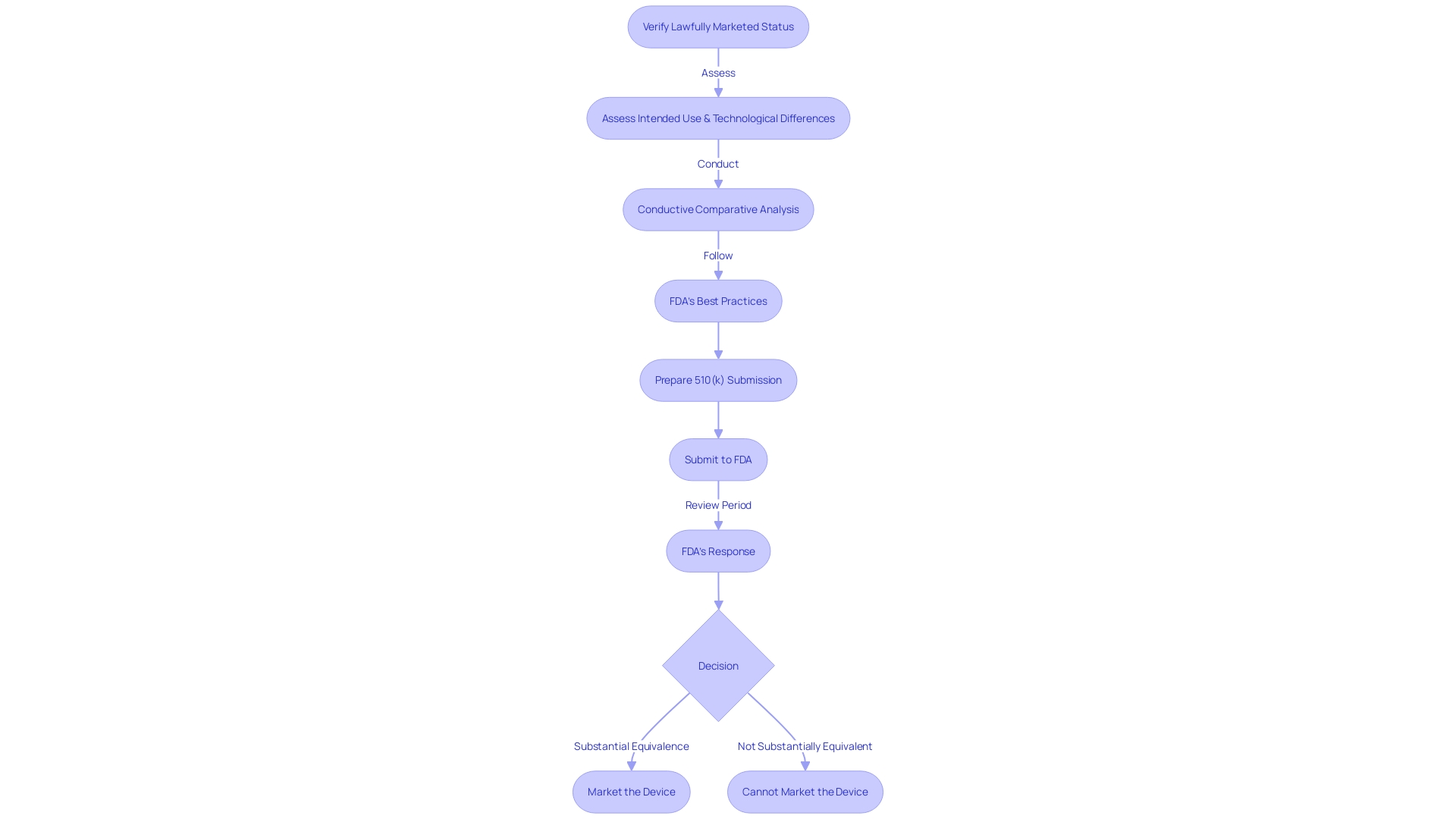
The 510(k) process allows for the clearance of medical devices that demonstrate substantial equivalence to a predicate device—a legally marketed device that serves as a comparison point. To establish this equivalence, it is crucial to conduct thorough research on the subject device, understanding its intended user base—whether clinicians, physicians, dentists, or patients—and carefully reviewing the instructions for use, including any warnings and precautions.
In collaboration with marketing teams, it's also advisable to analyze the competitive landscape, examining research literature, clinical studies, and promotional materials of competitor devices. This helps in identifying potential predicate devices with similar intended uses and technological characteristics. Creating a comparative table to juxtapose your device with these predicates can be a useful tool.
Once potential predicates are identified, consulting the Summaries of Safety and Effectiveness Data (SSED) available in the FDA’s 510(k) database is a critical step. These summaries provide detailed information on the safety and effectiveness of approved devices and can guide the development of a robust 510(k) submission.
Moreover, the FDA underscores the importance of transparency in the submission process. Any comments or documents submitted to the FDA may be made public, so it is incumbent upon the submitter to ensure that no confidential or sensitive information is inadvertently disclosed. This level of diligence and understanding of the FDA's processes and requirements is indispensable for regulatory professionals navigating the complex landscape of medical device approval.
Why is the FDA 510(k) Search important?
Navigating the complexities of FDA regulations is a critical task for any medical device manufacturer seeking market entry in the United States. One of the key steps in this process is understanding the various classification levels assigned by the FDA, which are based on the potential risk to patients. These classifications determine the appropriate registration pathway for a device, be it Premarket Notification (510(k)), Premarket Approval (PMA), or the De Novo process, each with distinct requirements and implications for market access.
With a 510(k) submission, manufacturers must demonstrate that their device is substantially equivalent to an existing device that has already been legally marketed - a process that confirms safety and effectiveness without the need for premarket approval. This is essential for devices that do not require the more rigorous PMA pathway, which is typically reserved for high-risk devices that have no previous predicate.
The FDA's database is an invaluable resource for manufacturers to examine regulatory histories, indications for use, and technological characteristics of medical devices. Such information guides comparative analyses, underpins informed decision-making, and upholds the safety and effectiveness standards of new devices. As the industry evolves, and with the advent of digital health technologies, regulatory guidance continues to adapt, ensuring that innovation is not stifled while maintaining patient safety.
For those involved in the production and distribution of medical devices targeting the U.S. market, annual establishment registration with the FDA is mandatory. This includes listing devices and detailing activities performed on them, a process made more efficient through the FDA's searchable databases. These databases, updated quarterly, include Postmarketing Requirements (PMRs) and Commitments (PMCs), providing transparency on ongoing and completed studies related to clinical safety and efficacy.
As the industry faces various obstacles, including regulatory challenges that can solidify the dominance of incumbent products, it is important to remember the lessons learned from other sectors. Similar to the strategic approaches taken by ride-sharing companies like Uber and Lyft to overcome market entry barriers, some medical device manufacturers have effectively navigated the regulatory landscape by creating sufficient market demand, although this must be balanced against the imperative of patient safety.
In conclusion, the FDA 510(k) Search and related databases serve as a cornerstone for the medical device industry, offering critical insights essential for compliance and success in a highly regulated market.
Navigating the FDA 510(k) Search
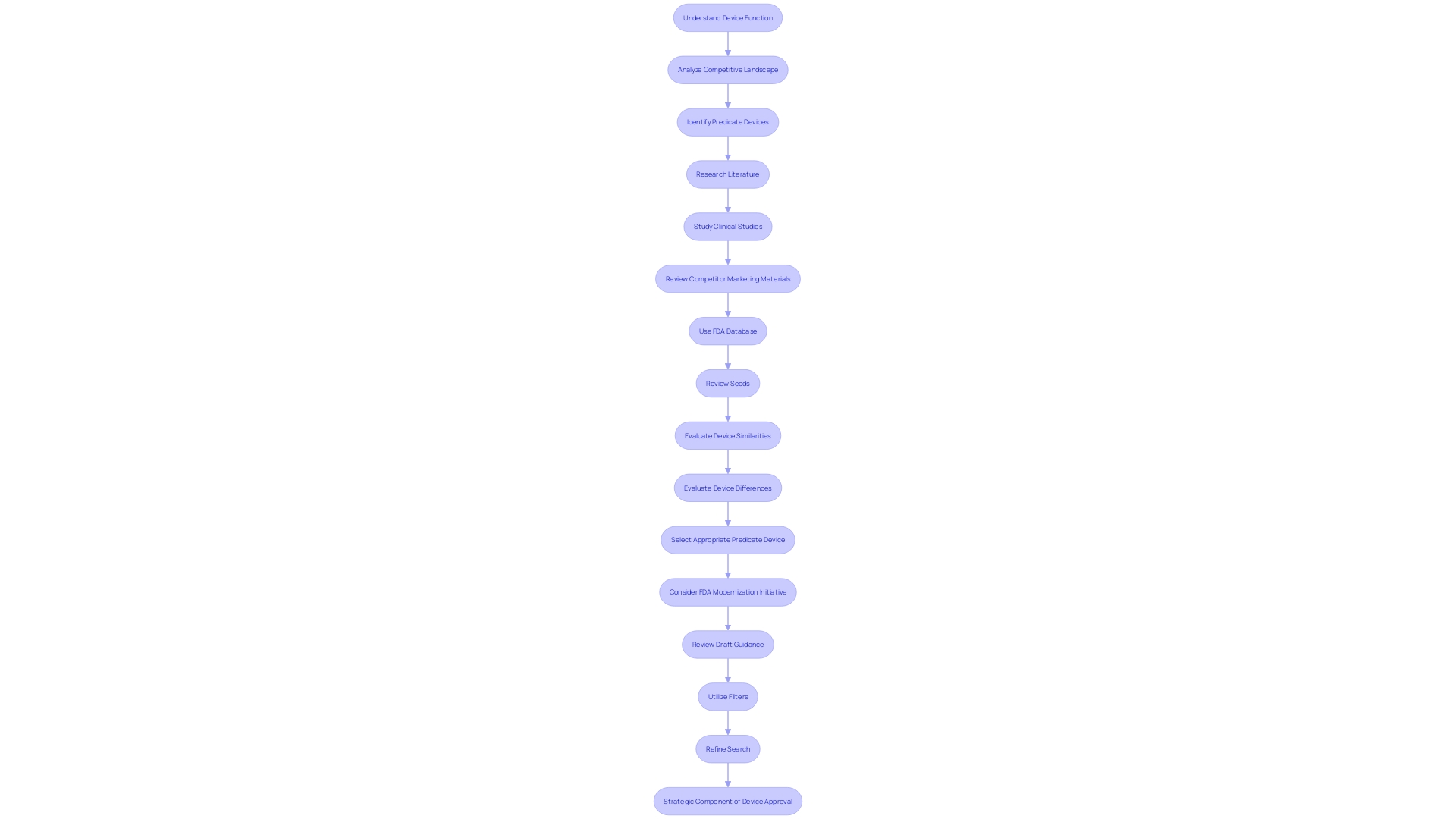
Mastering the FDA 510(k) Search is a critical aspect of medical device market authorization. Initiating this process by deeply understanding the device in question is paramount. This entails not just knowing the device's function but also grasping the intricacies of its use—cautions, warnings, and the spectrum of users from clinicians to patients. It’s equally important to analyze the competitive landscape, which helps in identifying potential predicate devices that share the same intended use and technological characteristics.
The insights gleaned from research literature, clinical studies, and competitor marketing materials, such as brochures and labeling, are invaluable. They serve as a foundation for creating a comparative table that juxtaposes your device against existing ones. The FDA’s database is an indispensable resource here, offering access to Summaries of Safety and Effectiveness Data (Seeds), which are crucial for evaluating the similarities and differences among devices.
With the FDA’s modernization initiative, the selection of an appropriate predicate device has become a nuanced process. It involves confirming that the potential predicate is not just legally marketed but also shares the same intended use without raising new safety or efficacy concerns due to differing technological characteristics.
The FDA, integral to public health, sets standards for the safety, efficacy, and security of medical devices. Their recent draft guidance, released in September 2023, underscores their commitment to streamlining the review process and ensuring that older predicates, with their extensive safety data, are utilized effectively.
In practice, utilizing the FDA 510(k) Search means employing all available filters—product code, device class, clearance date—and keywords to refine your search. Advanced search functionalities are also at your disposal to yield more precise results. However, it’s the preliminary groundwork of understanding both the device and its context in the market that transforms a simple search into a strategic component of device approval.
Understanding the Search Results
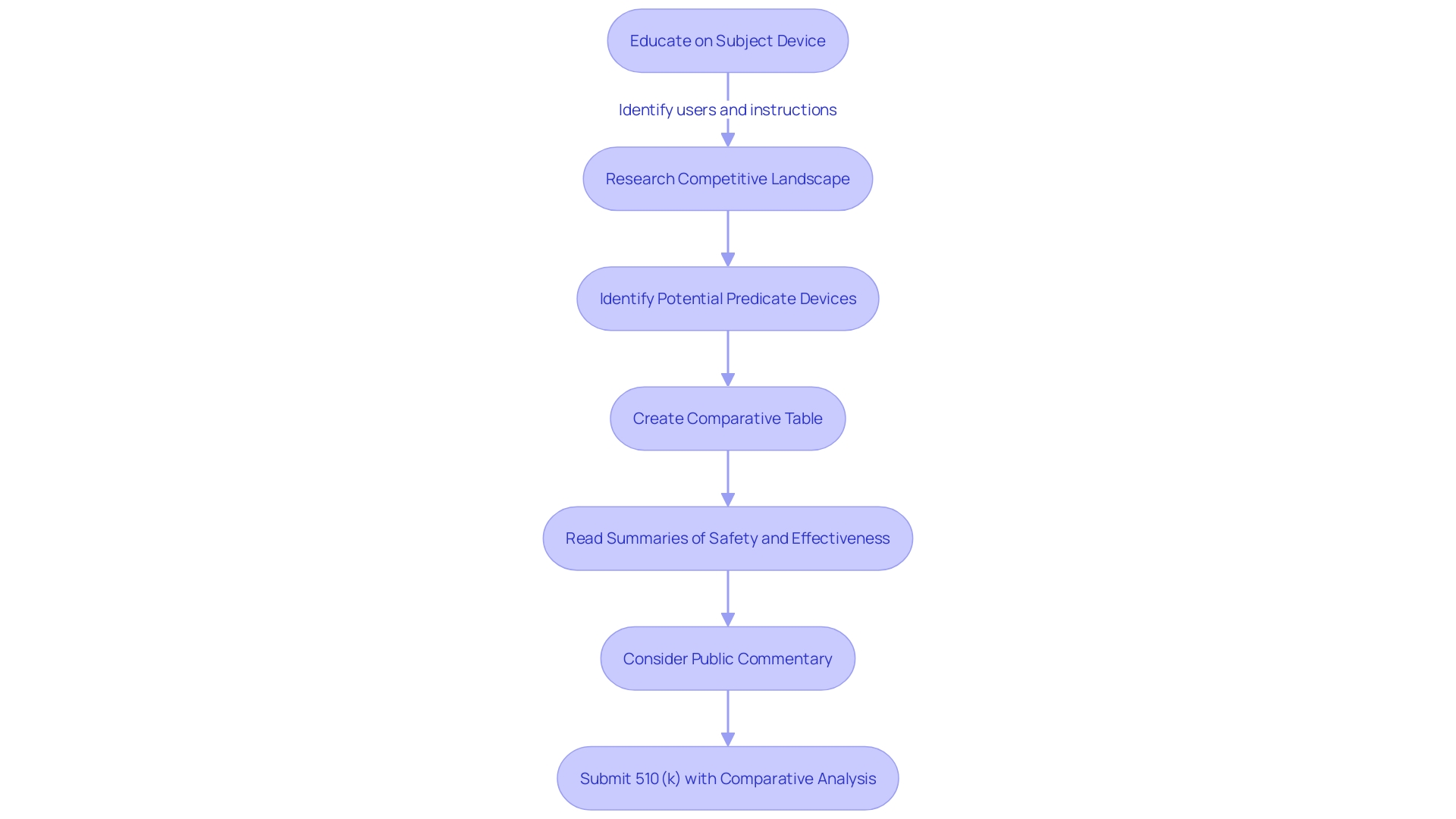
Utilizing the FDA 510(k) Search tool yields a list of medical devices that align with specified search parameters. This encompasses essential data like the device's designation, its manufacturing entity, date of clearance, and its recommended applications. For a more granular perspective, one can delve into individual device entries to unearth comprehensive details, such as the 510(k) summary, the associated FDA product code, and supplementary materials or documentation that support the device's approval.
The importance of comprehending a device's function and its application cannot be overstated, particularly for users such as clinicians, physicians, dentists, and patients. Equally critical is acquiring knowledge about the competitive environment of the device, which involves examining a plethora of resources, including research papers, clinical trial findings, and marketing materials. This aids in identifying analogous predicate devices featuring similar technological characteristics and intended uses, ultimately facilitating the construction of a comparative analysis table.
Moreover, the FDA's role extends beyond the database, as it ensures the safety, efficacy, and security of various health-related products, impacting public health on multiple fronts. For those intent on submitting public comments regarding devices, caution is advised to avoid including personal or confidential data that is not intended for public disclosure. The FDA's guidance on comment submission is a testament to its commitment to transparency and public engagement.
In the realm of healthcare innovation, the FDA's Center for Drug Evaluation and Research (CDER) plays a pivotal role in ushering new therapeutic options to the market, providing clear guidelines on study designs and data requirements for drug applications. This is crucial for the advancement of healthcare, offering novel treatments that address unmet medical needs. An annual compilation of newly approved molecular entities and therapeutic biological products is testament to CDER's significant contribution to medical progress.
Analyzing the 510(k) Summary
A crucial aspect of the FDA 510(k) submission process is the 510(k) summary, which is pivotal in the FDA's public database. This summary is not merely an overview; it's an essential comparison tool that delineates the similarities and differences between the new medical device and a previously cleared predicate device. To truly comprehend a device's safety, efficacy, and technological attributes, one must delve into the Summaries of Safety and Effectiveness Data (SSEDs), which provide insights into clinical performance and user considerations, such as indications, contraindications, and warnings.
Understanding the competitive environment is also beneficial. By analyzing research literature, clinical studies, and competitor marketing materials, stakeholders can identify predicate devices and forge a comparative analysis. This process is augmented by the FDA's recent draft guidance on best practices for predicate selection, as part of its initiative to modernize the 510(k) review process. The guidance emphasizes the importance of confirming that a potential predicate is legally marketed and shares the same intended use without introducing new safety concerns due to different technological characteristics.
Moreover, the FDA's Center for Drug Evaluation and Research (CDER) plays a fundamental role in ensuring the safety and effectiveness of new drugs and biological products, reflecting the agency's dedication to advancing healthcare. These efforts underscore the meticulous scrutiny and regulatory oversight that goes into every medical device reaching the market, safeguarding the well-being of the American public.
Utilizing the Predicate Device Information
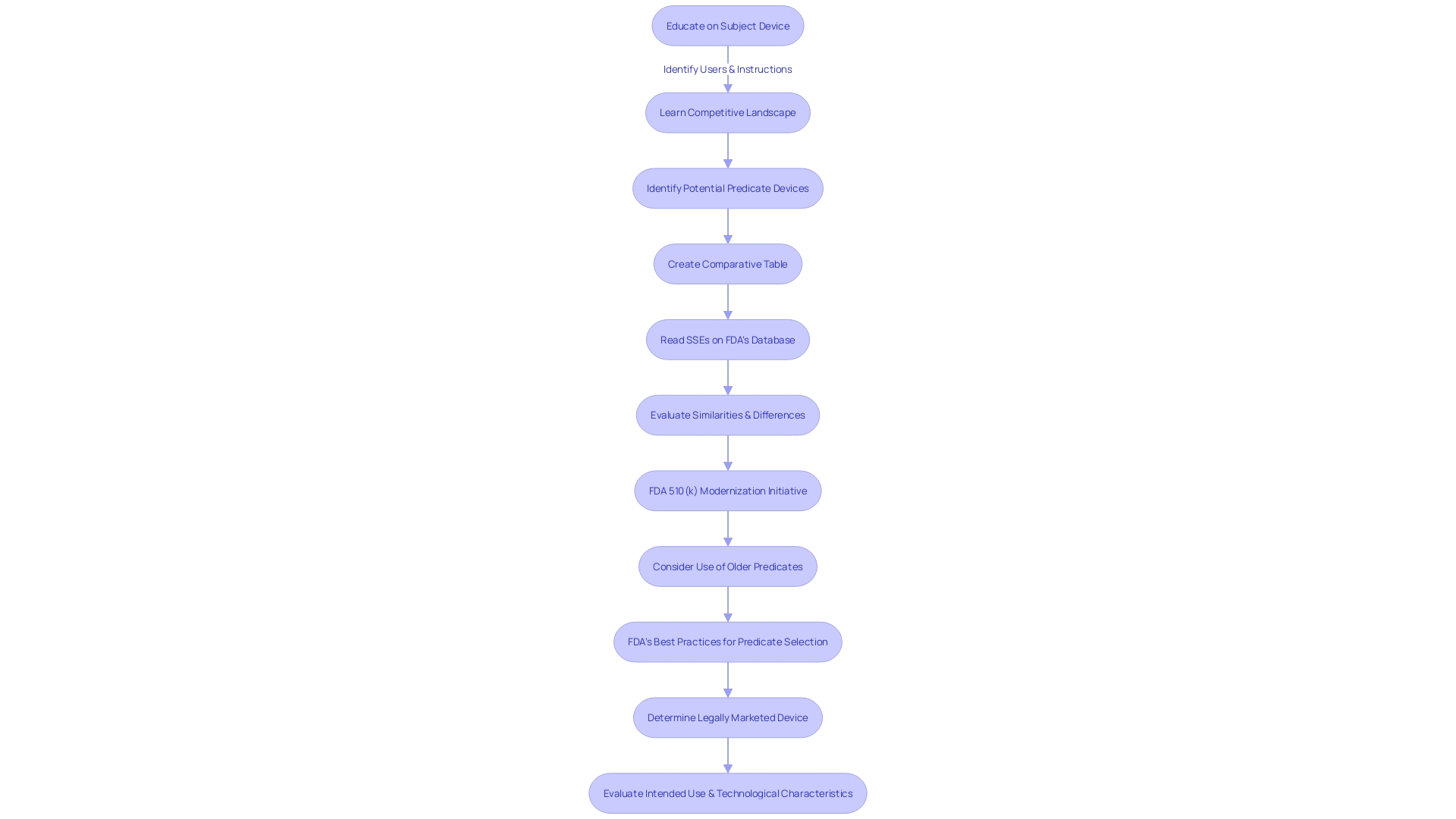
Navigating the FDA 510(k) submission process requires a keen understanding of predicate devices. Identifying similar devices and comparing their technological characteristics is a fundamental step towards ensuring that a new device aligns with existing safety and effectiveness benchmarks. A thorough examination of the predicate device's history, including its intended use and any improvements or alterations in technology, guides the developer in establishing a clear regulatory pathway for their device.
To illustrate, consider the case of a firm that recently revised its complaint coding and quality data analysis procedures. The firm is conducting a comprehensive review of historical complaints to ensure accurate coding, a process that is still underway. This reflects the meticulous nature of regulatory compliance and the importance of precise documentation when it comes to FDA submissions.
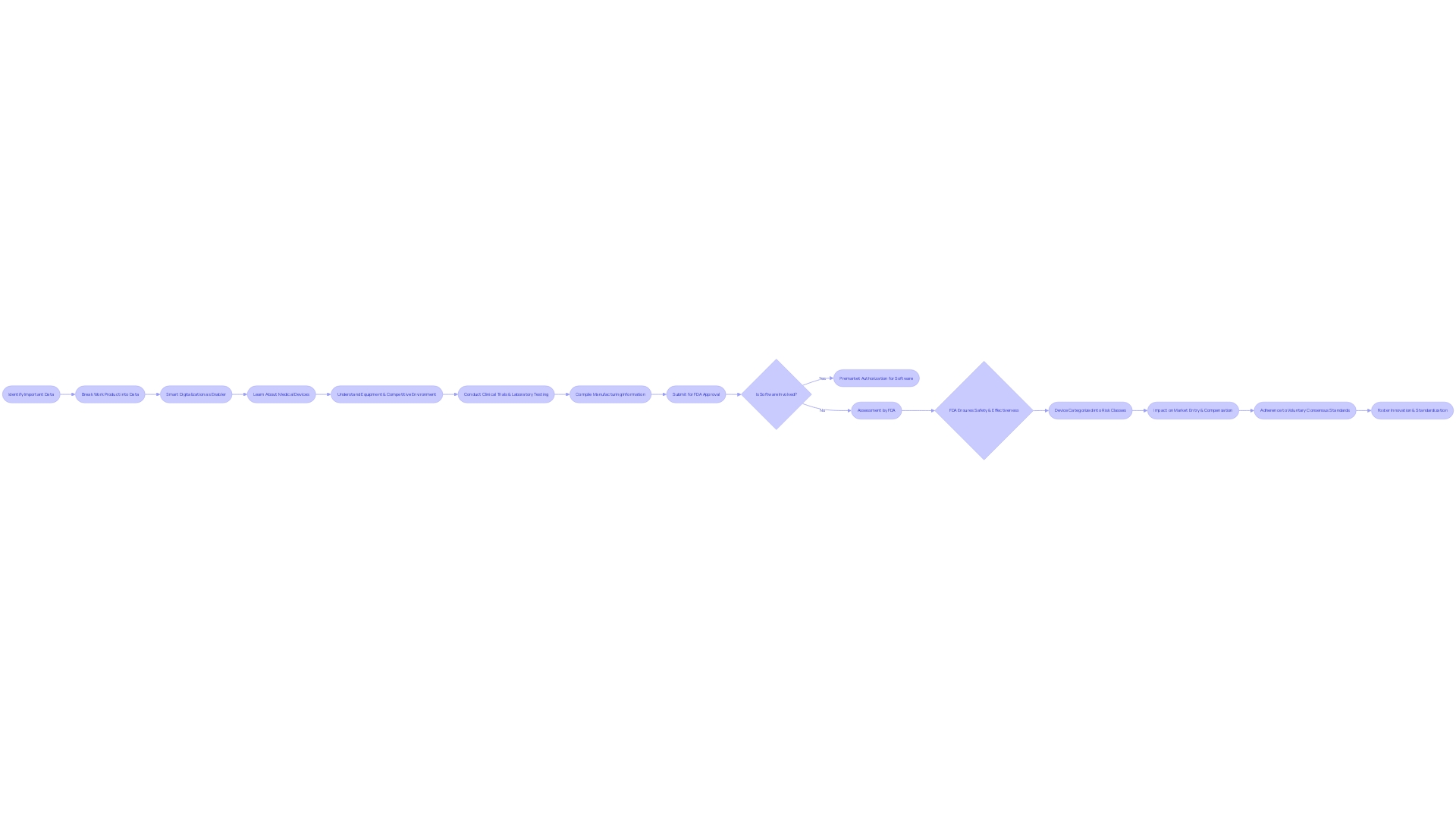
For example, the FDA's scrutiny of the Impella Connect System, which includes both software and hardware components, demonstrates the critical nature of software functions in medical device evaluation. The system's alarm notifications and real-time case status displays are considered device functions that require premarket authorization.
The FDA's 510(k) modernization initiative further underscores the importance of strategic predicate selection. The initiative encourages the use of older predicates when they offer benefits, such as extensive safety data. A draft guidance document released on September 7, 2023, provides best practices for choosing a predicate, emphasizing the need for a legally marketed device with a similar intended use and technological profile that does not introduce new safety concerns.
Understanding the classification level of the device in question is also crucial. The FDA categorizes medical devices into three distinct risk-based classes, each dictating a specific regulatory pathway—be it 510(k) Premarket Notification, Pre-Market Approval (PMA), or the De Novo process. The terminology—Registered, Cleared, Approved, and Granted—carries specific meanings and implications in the context of medical devices.
The FDA recently introduced a draft guidance detailing circumstances in which clinical data might be necessary for a 510(k) submission. This guidance elaborates on scenarios where non-clinical testing alone may not suffice to establish substantial equivalence with a predicate device. Such scenarios include differences in indications for use or technological characteristics, situations where non-clinical testing cannot determine substantial equivalence, or when new risks associated with the predicate device emerge.
Finally, the De Novo process stands as a pathway for novel devices without a legally marketed predicate. Success in the De Novo classification process not only classifies the device under a new regulatory category but also establishes it as a predicate for future submissions.
By delving into the intricacies of the 510(k) process and leveraging regulatory data, one can gain insights into the dynamics of medical device approval and the critical role of predicate device analysis in ensuring public health and safety.
Additional Resources and Databases
Beyond the FDA 510(k) Search tool, professionals seeking a deeper understanding of medical devices have access to several other critical resources. The FDA's Device Approvals and Clearances database, for instance, is instrumental in providing up-to-date information on the latest medical devices that have been authorized for use. The Medical Device Recall database offers valuable insight into products that have been withdrawn from the market due to safety concerns, echoing the lessons from the 2018 documentary The Bleeding Edge, which highlighted the potential risks associated with fast-tracked devices lacking clinical trial data.
The Manufacturer and User Facility Device Experience (MAUDE) database is another important resource that collects reports of adverse events involving medical devices. This database supports the FDA's post-market surveillance efforts, ensuring that any emerging issues with medical technologies are identified and addressed promptly.
For those navigating the regulatory frameworks and classifications of medical devices, understanding the distinction between terms such as 'Registered', 'Cleared', 'Approved', and 'Granted' is crucial. Each term corresponds to a different status within the FDA's approval process and determines how a device can be marketed and utilized within the US healthcare system.
Moreover, the alignment of FDA approval data with the requirements of payors, such as CMS and private health plans, is a significant factor in the availability of medical devices to patients. The information necessary for FDA clearance may not always fulfill the criteria needed for coverage determinations, leading to potential delays or denials in patient access to new medical technologies.
Dr. Yukiko Nakatani from the WHO emphasizes the need for centralized information sources to aid global decision-makers in the selection and procurement of medical devices. The expansive range of medical devices, now exceeding 10,000 different types, and their varying levels of complexity necessitate a comprehensive database such as MeDevIS. This platform allows for streamlined access to information on medical devices, supporting healthcare systems from community to specialized hospitals, and facilitating improved patient outcomes, especially in resource-limited settings.
Navigating the plethora of medical devices and their regulatory status is a complex task. However, with the right tools and databases at their disposal, professionals in the field can ensure that they are making informed decisions that prioritize patient safety and the efficacy of medical technologies.
Conclusion
The FDA's 510(k) premarket notification system is vital for medical device manufacturers entering the US market. Understanding the FDA's classification system and different levels of regulatory scrutiny is crucial. Thorough research, competitive analysis, and consulting the FDA's 510(k) database are essential steps.
The FDA 510(k) Search is indispensable for regulatory professionals, ensuring compliance and success. It allows clearance of devices demonstrating substantial equivalence to a legally marketed device. The database provides information on regulatory histories and technological characteristics, guiding comparative analyses.
Mastering the FDA 510(k) Search involves understanding the device, analyzing the competitive landscape, and utilizing search filters. The FDA's database supports informed decision-making and upholds safety and effectiveness standards.
Additional resources like the Device Approvals and Clearances database and the Manufacturer and User Facility Device Experience (MAUDE) database provide valuable insights. Understanding terms like 'Registered', 'Cleared', 'Approved', and 'Granted' is crucial for marketing and utilization within the US healthcare system.
In conclusion, the FDA 510(k) Search and related resources are vital for the medical device industry. Navigating FDA regulations, understanding classification levels, and utilizing available tools are key to success and ensuring patient safety.




