Introduction
The process of developing new pharmaceutical compounds and bringing them to market involves several critical steps and regulatory requirements. One of these crucial steps is obtaining Investigational New Drug (IND) status, which allows for the testing of the compound in humans through clinical trials. This article explores the concept of IND, the different types of IND applications, the IND application process, key components of an IND application, the role of IND in clinical trials, ethical considerations in IND research, and the challenges and limitations of IND studies.
By delving into these topics, we gain a comprehensive understanding of the complex journey from drug development to market authorization and the importance of IND in advancing medical science and improving patient outcomes.
What is an Investigational New Drug (IND)?
The term Investigational New Drug (IND) refers to a pharmaceutical compound that has been permitted by regulatory agencies, like the FDA, to be tested in humans. Obtaining IND status is a pivotal step that is preceded by the submission of significant preclinical findings, including safety and efficacy profiles. Once approved, an IND permits the start of clinical trials, enabling researchers to systematically gather data on the medication's therapeutic effects, appropriate dosages, and potential adverse reactions.
To ensure the safety and effectiveness of these investigational substances, strict regulations are in place. These include extensive documentation covering the product's composition, manufacturing entities, and detailed descriptions of the manufacturing and packaging processes. The specifications must encompass all aspects necessary to maintain the medication's identity, strength, quality, purity, potency, and bioavailability. This involves establishing criteria for sterility, dissolution rates, and container closure systems, supported by stability data that inform expiration dating.
Manufacturers are accountable for complying with all federal laws and FDA regulations, understanding that non-compliance can lead to serious legal repercussions, including seizure and injunction. It's essential for firms to provide prompt, written communication to the FDA detailing corrective actions taken in response to any concerns raised, ensuring violations do not recur.
Innovative pharmaceuticals and biological products are vital for advancing healthcare and offering new treatment avenues to patients. The FDA's Center for Drug Evaluation and Research (CDER) aids pharmaceutical developers by clarifying necessary study designs and data requirements for medication applications. Every year, CDER approves a variety of new medications and biological products, some of which are innovative to medical practice, while others provide alternative choices to current therapies.
The pursuit of medication approval is motivated by the need to address unmet health needs. Market potential is a significant factor for companies like Novartis, which prioritize development based on the scale of the health market. Effectiveness and safety are crucial, yet they are complex concepts that depend heavily on the context of each product's intended use, as determined by clinical experimentation designs and the indications for which the product is licensed.
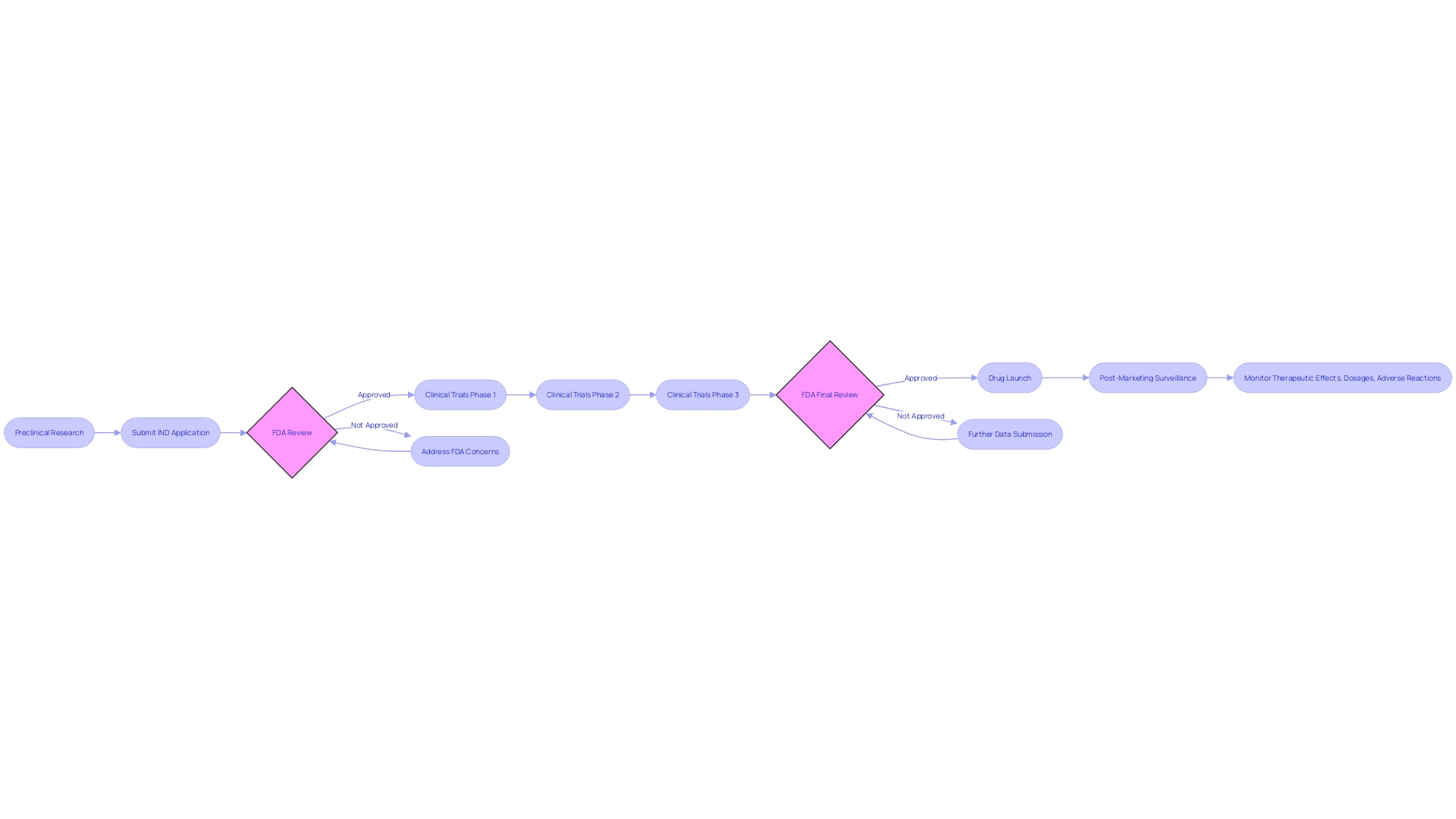
Types of IND Applications
The scenery of Investigational New Drug (IND) applications is complex, with various types addressing different stages of medication development and purposes. Three principal types of INDs include:
-
Investigator-Initiated IND: Frequently submitted by researchers or institutions, this IND is for individuals who have developed a unique pharmaceutical or biologic and are seeking to carry out experiments to assess its safety and effectiveness. For instance, Cardinal Health's partnership with a client to navigate the regulatory complexities for their IND submission demonstrates the intricate process involved in an investigator-initiated application.
-
Sponsor-Initiated IND: Typically submitted by pharmaceutical companies or sponsors, this application is a milestone towards the development and eventual commercialization of a medication. These sponsors are responsible for starting clinical investigations to acquire approval. A noteworthy example is Verve Therapeutics' strategic approach to addressing hypercholesterolemia, illustrating the sponsor's role in advancing treatment options through the IND process.
-
Emergency Use IND: This application is reserved for dire circumstances where a novel drug is urgently needed to treat life-threatening conditions. The FDA can expediently grant an Emergency Use IND to facilitate access to critical treatments.
Clinical experiments, pivotal to the advancement of these applications, are the bridge between emerging research and the introduction of new treatments to the public. They encompass everything from new pharmaceuticals to surgical procedures and require volunteers for each phase to advance potential discoveries. The National Institutes of Health (NIH) explains that assessments conducted in a medical setting are research efforts aimed at evaluating the influence of interventions on health outcomes. As an instance, the phases of medical experiments for diabetes treatments demonstrate the comprehensive process each potential therapy undergoes before FDA approval.
The end goal is to ensure the safety and efficacy of new therapies. Drug development traverses several stages, each meticulously overseen by regulatory bodies to protect public health. Both the IND and New Drug Application (NDA) are crucial junctures in this journey, guiding a medication candidate from preclinical stages through clinical trials to market authorization. It's important for developers in the pharmaceutical industry to understand which data are necessary for each application and when to submit it.
Despite the staggering costs and time investments associated with pharmaceutical development—often exceeding a billion dollars and spanning over a decade—the commitment to advancing medical science persists. Clinical experiments, increasing in intricacy, have a notable impact on this financial and temporal investment, with only a portion of medications advancing past the initial phase ultimately obtaining approval. Insights from the IQVIA Institute for Human Data Science indicate that, despite the challenges, the commitment to research and development in the biomedical field remains robust, driven by the potential to transform healthcare.
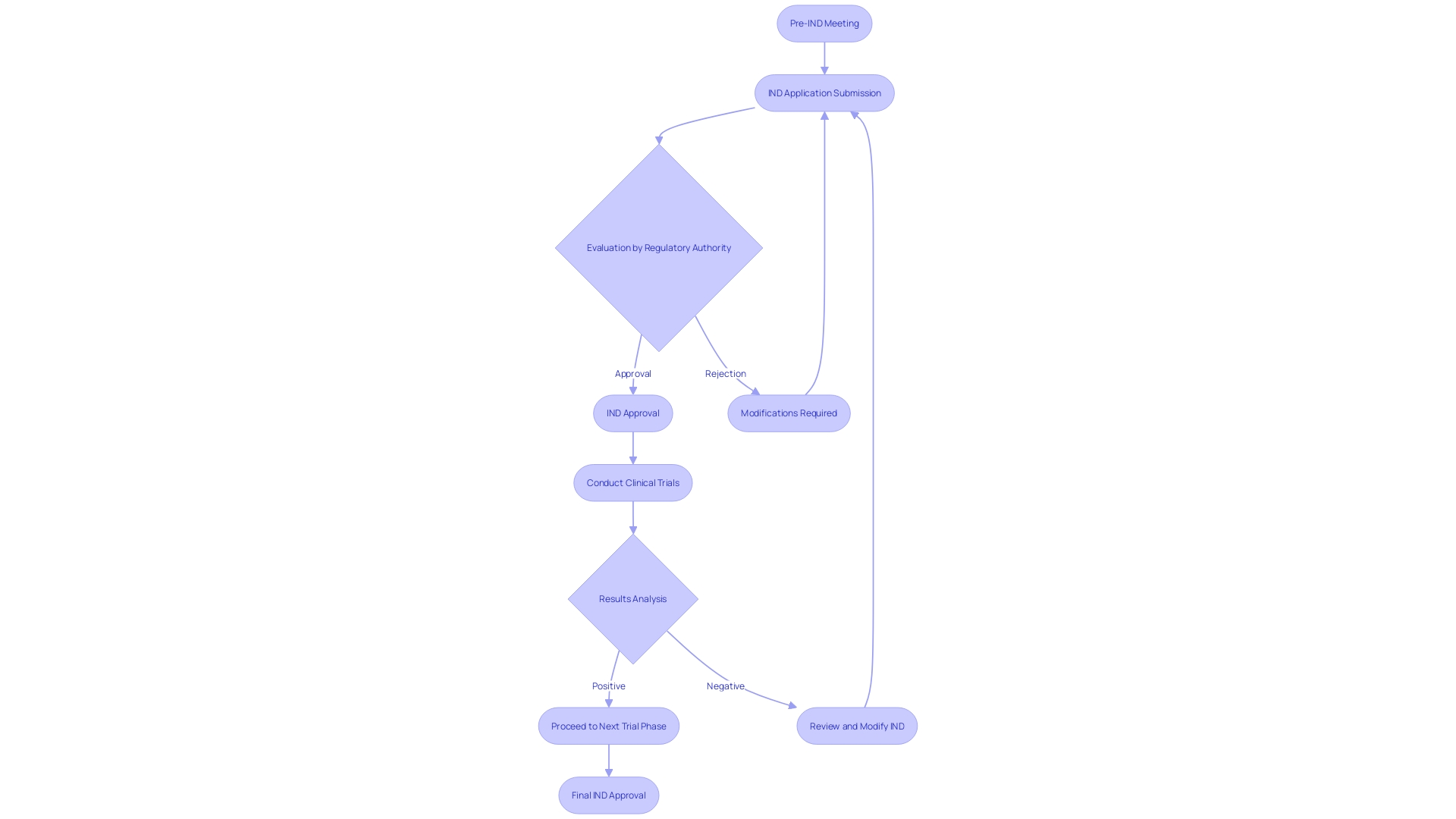
IND Application Process
Navigating the investigational new medication (IND) application process is a critical step in the development of new pharmaceuticals. It starts with a pre-IND meeting, a collaborative discussion with regulatory authorities to clarify the study design and regulatory expectations. This proactive approach can uncover any existing solutions within the institution, as seen in the digital technology adoption within the NHS, and ensures the proposed study aligns with current safety standards and regulatory frameworks.
After this, a comprehensive IND application is submitted, detailing the medication's composition, manufacturing processes, and preclinical data, mirroring the thorough documentation required for medication product manufacturing as highlighted by the U.S. Pharmacopeia. The regulatory authority conducts a thorough evaluation of this application, examining the safety and potential effectiveness of the drug, relying on the preclinical information and suggested trials. It is a pivotal step, akin to the scrutiny of safety standards applied to investor-owned electrical companies, ensuring the risk-benefit profile is favorable.
After meeting the regulatory criteria, IND approval is given, allowing the start of medical experiments. This echoes the forward-looking statements from companies like Intellia, outlining their commitment to advancing investigational gene editing therapies through robust programs.
During the progression of the experiments, modifications to the IND may be required to improve research protocols or address safety concerns. These changes must be approved by the regulatory authority, ensuring ongoing compliance and patient safety. This adaptable approach is essential, especially considering the dynamic nature of development and the need for speed to market, underscored by the recent analysis from McKinsey showing that the majority of trials do not finish on time. Ensuring an agile yet compliant IND process is paramount to advancing medical science and ultimately improving patient outcomes.
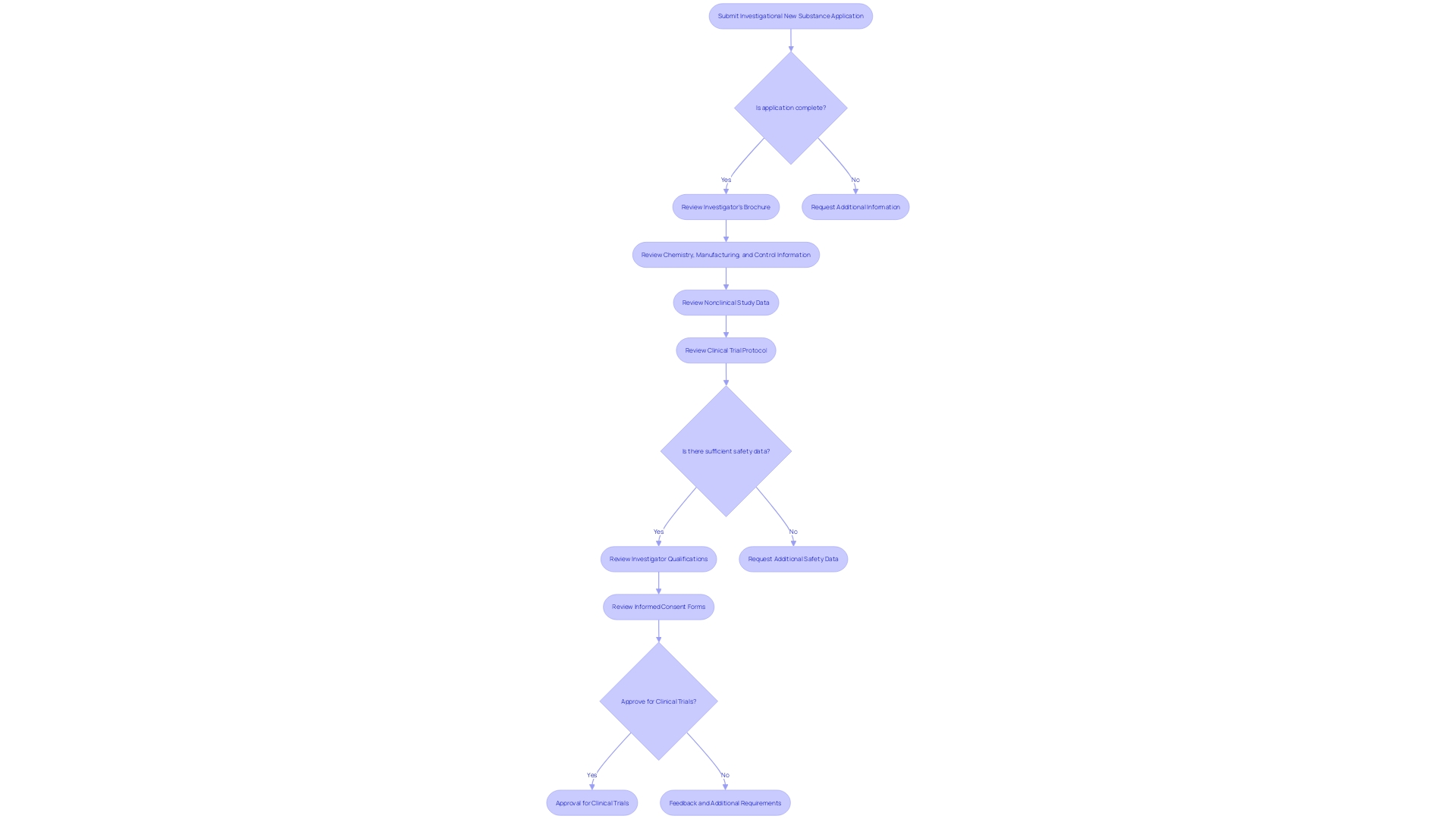
Key Components of an IND Application
An Investigational New Substance (INS) application is a crucial stage in the substance development process, where comprehensive information about the investigational substance and the proposed studies is submitted to regulatory authorities. The application is composed of several essential elements:
-
The Investigator's Brochure, which encapsulates the medication's profile, including its chemical structure, pharmacological and toxicological data, and insights from any prior clinical experiences. This document serves as an essential point of reference for both investigators carrying out the experiments and the regulatory bodies supervising the progress of the medication.
-
Chemistry, Manufacturing, and Control (CMC) Information is included to outline the medication's synthesis, quality assurance measures, and stability profiles, ensuring the medication's identity, strength, and purity remain consistent throughout its production.
-
Data from Nonclinical Studies, which are indispensable as they provide early safety signals and pharmacokinetic profiles from preclinical testing, often utilizing animal models to predict human responses.
-
The Clinical Trial Protocol details the blueprint of the study, stating its goals, methodologies, participant criteria, dosage plans, and the procedures for data collection and analysis.
-
Safety Data, which consists of any existing study results that emphasize the drug's safety profile, along with a comprehensive approach for monitoring and reporting any adverse events during the experiments.
-
Investigator Qualifications, where the credentials and expertise of the research study investigators are presented to affirm their capability in conducting the study effectively.
-
Informed Consent Forms are carefully designed to convey the study's objectives, approaches, possible hazards and advantages, and the participants' entitlements, guaranteeing that subjects are completely informed and willing to accept the terms of the study.
These components together create a strong framework for the IND application, providing a comprehensive portrayal of the investigational medication and its intended evaluation in a medical setting, thus allowing regulatory bodies to carefully evaluate its potential for safe and efficient utilization.
The Role of IND in Clinical Trials
Navigating the intricate landscape of clinical trials is a multifaceted endeavor, where the Investigational New Drug (IND) application emerges as a cornerstone, framing the initiation and conduct of clinical studies. The IND status not only allows the administration of experimental medications to humans but also outlines a structured approach to gather and evaluate data regarding the medication's safety, dosage, efficacy, and potential adverse effects. This information is indispensable in ascertaining whether the drug can effectively manage the targeted medical condition while ensuring that its therapeutic advantages surpass any associated risks.
The strategic use of quality registries, as demonstrated by the DAPA-MI study team, exemplifies the innovative amalgamation of real-world data with the rigorous controls of a randomized investigation. This blend aims to maximize patient enrollment in a pragmatic setting while economizing resources and enabling the extraction of robust efficacy data. The experiment's shift from the anticipated main result due to fewer-than-expected occurrences highlights the ever-changing character of medical investigation and the significance of flexible experiment structures.
Furthermore, transparency and patient-centric practices are essential to the process of conducting clinical experiments. For example, during radical prostatectomy research, patients are fully informed about the surgery and its technical uncertainties, with some aspects being randomized within the study. Here, patient involvement is further enhanced by utilizing a web-interface to collect baseline functional data, thereby integrating patient-reported outcomes directly into their medical records.
Amidst the complexities, the endeavor to streamline participation in experiments persists. Progress in digital platforms has made the search and qualification process for medical experiments easier, allowing patients to sort options by disease stage, location, and other criteria. This is vital in an era where research experiments have extended beyond medication safety and effectiveness to include investigative gene editing therapies, as observed with Intellia's NTLA-2001 for transthyretin amyloidosis. The company's forward-looking statements reveal a strategic commitment to start a global Phase 3 study by year-end 2023, yet they also acknowledge the myriad risks and uncertainties inherent in development.
Overall, the IND plays a crucial role in drug development, enabling a smooth transition from preclinical research to the testing phase. It mandates thorough preclinical data on pharmacology, toxicology, and manufacturing to satisfy regulatory requirements and ensure public safety. With a focus on patient engagement, adaptive methodologies, and the leveraging of technological advancements, the trials process continues to evolve, bearing the promise of novel therapeutic discoveries.
Ethical Considerations in IND Research
In the realm of Investigational New Drug (IND) research, ethical considerations are paramount, not only to safeguard the rights and welfare of study participants, but also to navigate the complex landscape of emerging technologies. A thorough ethical framework encompasses several critical components:
-
Informed Consent: It is imperative for participants to grant consent only after fully comprehending the study's objectives, potential risks and benefits, and their rights. This process is a cornerstone of ethical clinical research, affirming participant autonomy.
-
Risk-Benefit Analysis: Researchers must meticulously evaluate the drug's possible risks and benefits. The ethical conduct of an experiment is based on ensuring that the expected benefits for participants are greater than the potential risks.
-
Participant Privacy: Strict protocols must be in place to protect participant confidentiality, ensuring secure management of personal information and maintenance of anonymity.
-
Continual Monitoring and Safety Reporting: The researchers' duty extends to ongoing vigilance over participant safety, including the swift reporting of any adverse events to regulatory bodies.
-
Ethical Oversight: Every IND study involving human subjects is subject to review by institutional ethics committees, which assess whether the study's design and procedures adhere to established ethical standards.
A case study examining the governance of rapidly evolving health technologies underscores the importance of these ethical pillars. For instance, a scenario involving a quadriplegic individual who received a brain implant underscores the ethical quandary when the device malfunctions post-trial, and the manufacturer is no longer available to provide support. This raises significant ethical concerns about the responsibility of care post-trial, especially in the absence of legal mandates.
Statistical trends emphasize the timeliness and difficulties experienced by researchers, with up to 80 percent of experiments not finishing on schedule. The significance of timely completion of experiments is highlighted by the competitive aspect of therapeutic development and the urgent requirement for treatments for diseases with high unmet medical needs.
In light of these considerations, it is essential to recognize that ethical principles are not static but must evolve with the technology and the times. A document such as the AI Bill of Rights, which encapsulates core principles like data privacy and non-discrimination, serves as a beacon for researchers navigating the ethical dimensions of AI in biomedical research. As stated in recent analyses, the collective efforts of the research community continue to be driven by the immense potential to advance medicine, as evidenced by the sustained investment and numerous ongoing research programs globally.
By embracing a comprehensive and dynamic ethical framework, researchers can responsible conduct IND research that not only prioritizes the well-being and rights of study participants but also aligns with the evolving landscape of medical technology and governance.
Challenges and Limitations of IND Studies
Investigational New Drug (IND) studies are integral to medical progress, but they encompass a myriad of challenges and constraints. For example, the long period of time and significant financial commitment needed for these studies, from preclinical stages to completion of testing, can be especially challenging for smaller organizations or those with limited budgets.
Regulatory demands also present a formidable obstacle. The stringent and intricate guidelines governing IND studies necessitate meticulous compliance to secure and uphold IND authorization, a process that can be daunting, especially to newcomers in the field.
Another significant hurdle is the recruitment and retention of study participants. Conditions with limited patient populations or stringent eligibility criteria pose difficulties in amassing sufficient data, leading to potential delays or inconclusive results.
Safety concerns persist despite rigorous preclinical testing, with the possibility of unforeseen adverse events during clinical trials. This necessitates constant vigilance and immediate action to address any emergent issues, ensuring participant safety.
Furthermore, the limited sample size characteristic of IND studies may not reflect the broader population, thus constraining the generalizability of findings and necessitating additional research for validation across larger cohorts.
It is crucial for researchers to be cognizant of these challenges in order to proactively confront and surmount them, thereby contributing to the evolution of medical knowledge through valuable IND study insights.
Conclusion
In conclusion, the Investigational New Drug (IND) process is a crucial step in advancing medical science and improving patient outcomes. IND status allows for the testing of pharmaceutical compounds in humans through clinical trials, providing vital data on safety, efficacy, and potential adverse reactions. The IND application process involves rigorous documentation and compliance with regulatory requirements to ensure the quality and potency of the drug.
Different types of IND applications, including investigator-initiated, sponsor-initiated, and emergency use, address various stages and purposes of drug development. The IND application consists of key components such as the Investigator's Brochure, CMC information, nonclinical study data, the clinical trial protocol, safety data, investigator qualifications, and informed consent forms. These components provide a comprehensive overview of the investigational drug and its intended clinical evaluation, allowing regulatory bodies to assess its potential for safe and effective use.
The IND status not only authorizes the administration of experimental drugs to humans but also guides the collection and assessment of data in clinical trials. Ethical considerations, such as informed consent, risk-benefit analysis, participant privacy, continual monitoring, and ethical oversight, are vital to protect participant rights and welfare.
Despite challenges such as the extensive duration, financial investment, regulatory demands, recruitment and retention of participants, safety concerns, and limited sample size, researchers must proactively address these obstacles to contribute valuable insights to medical knowledge.
Overall, the IND process is a critical bridge from drug development to market authorization. By adhering to ethical principles, embracing technological advancements, and overcoming challenges, researchers can advance medical science and bring new treatments to patients. The commitment to research and development in the biomedical field remains strong, driven by the potential to transform healthcare and improve lives.




