Introduction
Clinical trials play a crucial role in advancing medical knowledge and improving patient care. However, ensuring the safety and integrity of these trials requires accurate and comprehensive reporting of adverse events. Serious Adverse Events (SAEs) are incidents with potentially grave outcomes that can occur during clinical studies and necessitate immediate reporting and investigation.
This article explores the importance of reporting SAEs, the classification and definition of adverse events, the role of platforms like ClinicalTrials.gov in reporting SAEs, the consequences of selective reporting, the role of Clinical Research Associates (CRAs) in monitoring and documenting adverse events, best practices for documenting and reporting adverse events, and initiatives to improve transparency and accessibility of adverse event data. The integrity of clinical research and the trust it commands from the public rely on transparent and accurate reporting, ultimately safeguarding patient health and advancing medical knowledge.
Background: Importance of Reporting Adverse Events in Clinical Trials
Understanding Serious Adverse Events (SAEs) in clinical trials is imperative for safeguarding participant health and maintaining the integrity of the research. SAEs are incidents with potentially grave outcomes that can arise during the course of a clinical study. These events necessitate immediate reporting and thorough investigation to assess whether they are linked to the intervention being tested.
Clinical researchers, such as Gamertsfelder, and organizations like the one led by Osipenko, are acutely aware of the personal sacrifices made by trial participants, particularly those with severe conditions like transthyretin-mediated amyloidosis. The elderly, who often manage multiple health issues, may undergo a battery of invasive tests and experience new side effects as they contribute to the advancement of medicine for future generations.
The importance of SAE reporting is further underscored by systems like the Vaccine Adverse Event Reporting System (VAERS), which has been instrumental in identifying vaccine safety issues. While VAERS is a post-marketing surveillance tool for vaccines, its principles of monitoring adverse events are relevant in the broader context of clinical trials where safety monitoring is crucial.
Participants' well-being relies on the rigorous documentation and timely communication of any SAEs, ensuring that researchers can take appropriate actions to mitigate risks. This not only protects current participants but also ensures that future clinical trials are conducted with the highest standards of safety, enhancing the credibility and reliability of the research outcomes.
Definition and Classification of Adverse Events
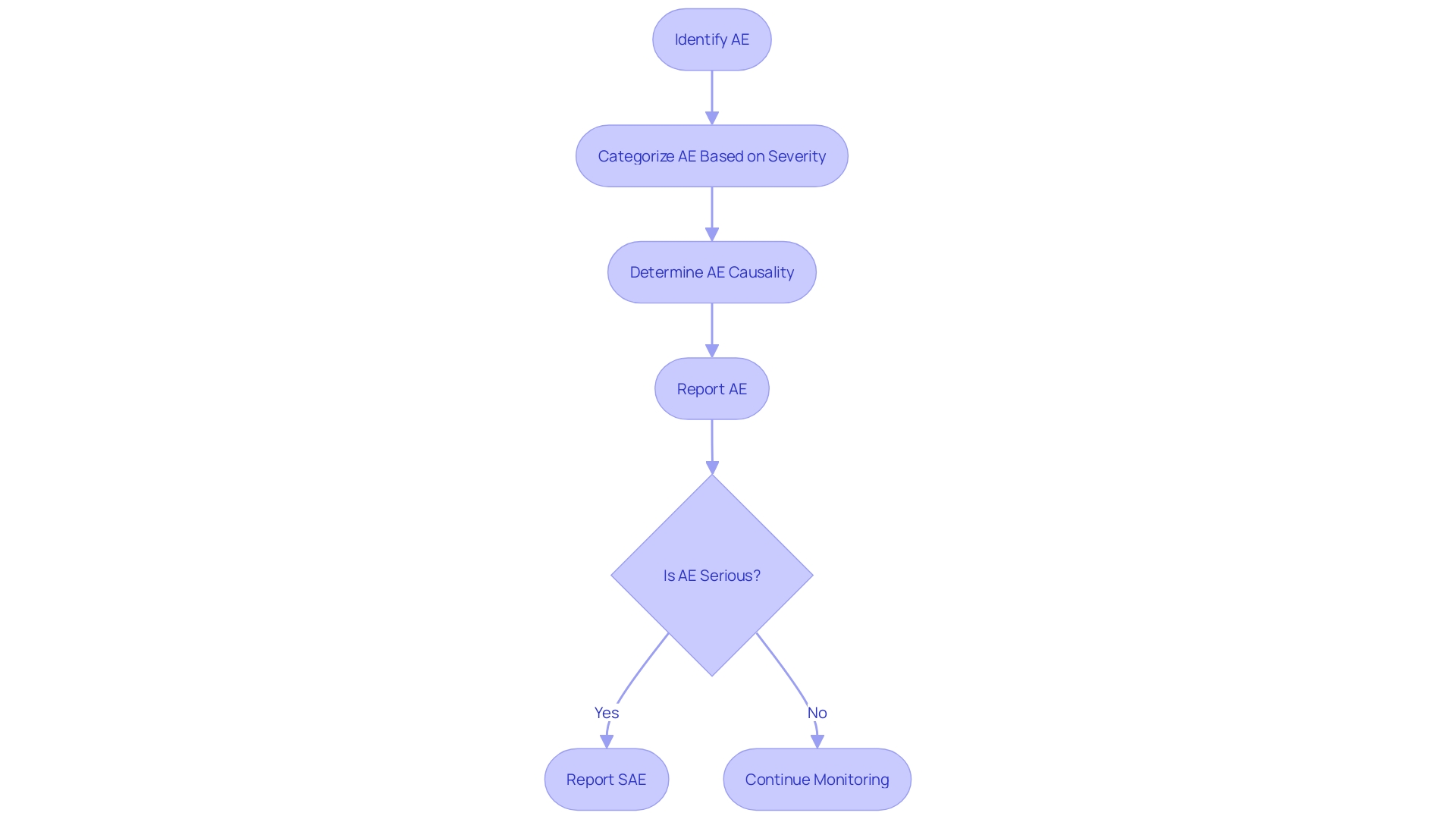
The classification of adverse events (AEs) in clinical trials is pivotal to ensuring the safety and efficacy of medical interventions. AEs are broadly categorized based on their severity, from mild to severe, and this gradation is critical when reporting serious adverse events (SAEs). It is the gradation of these events that informs the regulatory guidelines and ensures patient safety. For instance, the Vaccine Adverse Event Reporting System (VAERS) has been instrumental in identifying vaccine safety issues, underscoring the importance of a robust AE reporting system.
Clinical trials are the bedrock of establishing a product's efficacy, which is intrinsically linked to its designated indication as approved by the FDA. This indication is rooted in the clinical trial design and the subsequent outcomes measured. As noted in industry analysis, success in clinical trials, which is supported by human genetic evidence, has been associated with two-thirds of the drugs approved by the FDA in 2021. This highlights the significance of a systematic approach in clinical trial design and the reporting of results. Such approaches have been adopted to improve success rates and reduce attrition, which is often due to lack of efficacy or unforeseen safety issues.
To effectively report SAEs, researchers must navigate the complex landscape of AE classification, leveraging a standardized framework that is both comprehensive and nuanced. This allows for an accurate identification of SAEs, which is crucial for the advancement of medical science and the protection of public health. The process of determining the severity and causality of AEs is integral to this framework, ensuring that each event is appropriately captured and addressed. By focusing on these classifications and reporting mechanisms, researchers can contribute to the ongoing effort to enhance the safety profiles of medical interventions and uphold the highest standards of patient care.
The Role of ClinicalTrials.gov in Reporting Serious Adverse Events
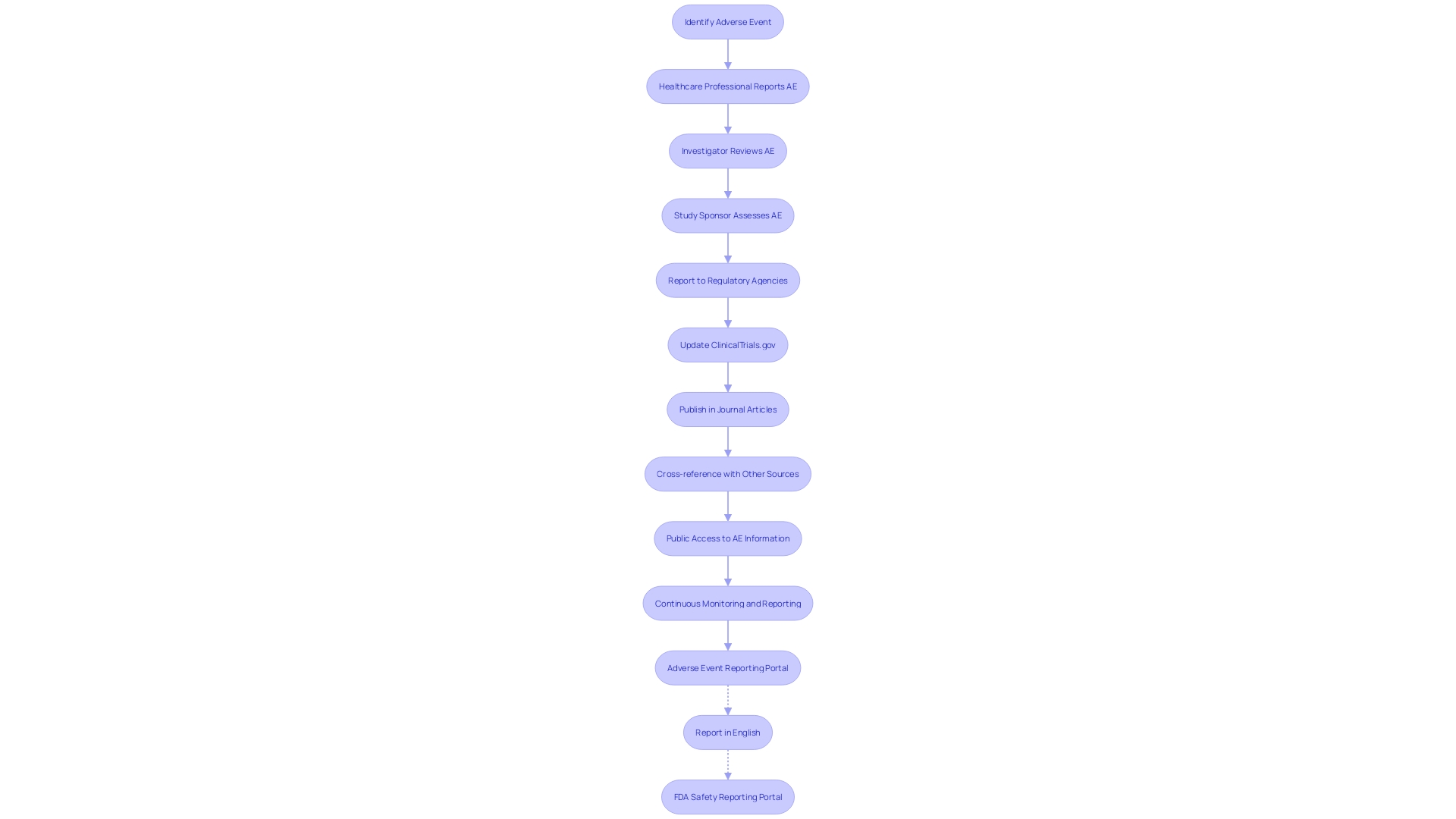
ClinicalTrials. Gov serves as an essential hub for enhancing the transparency and accountability of clinical research. It is a crucial resource for the reporting of Serious Adverse Events (SAEs), which are a key concern in patient safety and treatment efficacy. The platform mandates detailed reporting of adverse events, which is critical for the ongoing assessment of new treatments and interventions. This includes drugs, devices, and behavioral interventions, all undergoing rigorous evaluation to establish safety and effectiveness for patient use.
Through the database, researchers and healthcare professionals can access comprehensive data on adverse events, aiding in the critical analysis of the safety profile of interventions. For example, the FDA's Postmarketing Requirements and Commitments Searchable Database, which is updated quarterly, is publicly accessible and includes information on postmarketing studies and trials that focus on clinical safety and efficacy, among other areas. The platform's utility is further exemplified by the Manufacturer and User Facility Device Experience (MAUDE) database, maintained by the FDA, which collects reports of patient deaths, injuries, and device malfunctions.
ClinicalTrials.gov not only serves regulatory bodies and healthcare providers but also empowers patients and the public by providing accessible information on the risks associated with medical interventions. The narrative of each clinical trial participant, managing health conditions and enduring invasive tests, underscores the personal stakes involved in clinical research and the importance of meticulous adverse event reporting.
The role of ClinicalTrials.gov in reporting SAEs showcases a commitment to safeguarding patient health and optimizing treatment outcomes. It is a testament to the collaborative effort between researchers, regulatory agencies, and the public to maintain a high standard of medical care and research integrity.
Comparison of Adverse Event Reporting: ClinicalTrials.gov vs. Published Articles
The task of tracking and reporting adverse events (AEs) in clinical trials is a critical one, yet inconsistencies often exist between records in ClinicalTrials. Gov and published articles. Exploring the Yellow Card Scheme (YCS), an early warning system for unexpected adverse drug reactions founded in 1964, can shed light on the broader issue of AE reporting. The YCS, which allows public reporting of suspected AEs to the UK's Medicines and Healthcare Regulatory Agency (MHRA), underlines the value of public submissions in identifying potential pharmaceutical harms. A survey related to the YCS revealed that nearly half of the respondents discovered the reporting system through pharmacies, and 81% initiated their reports independently, emphasizing the public's role in contributing to drug safety vigilance.
The reporting practices on ClinicalTrials.gov have evolved over the past 25 years, with changes in data elements and requirements reflecting shifts in trial reporting laws and policies. This means older records might lack information that was not collected or was optional at the time of submission but is now required. On the modernized ClinicalTrials. Gov website, efforts have been made to clearly display the information submitted by study sponsors and investigators and to note what data has not been provided.
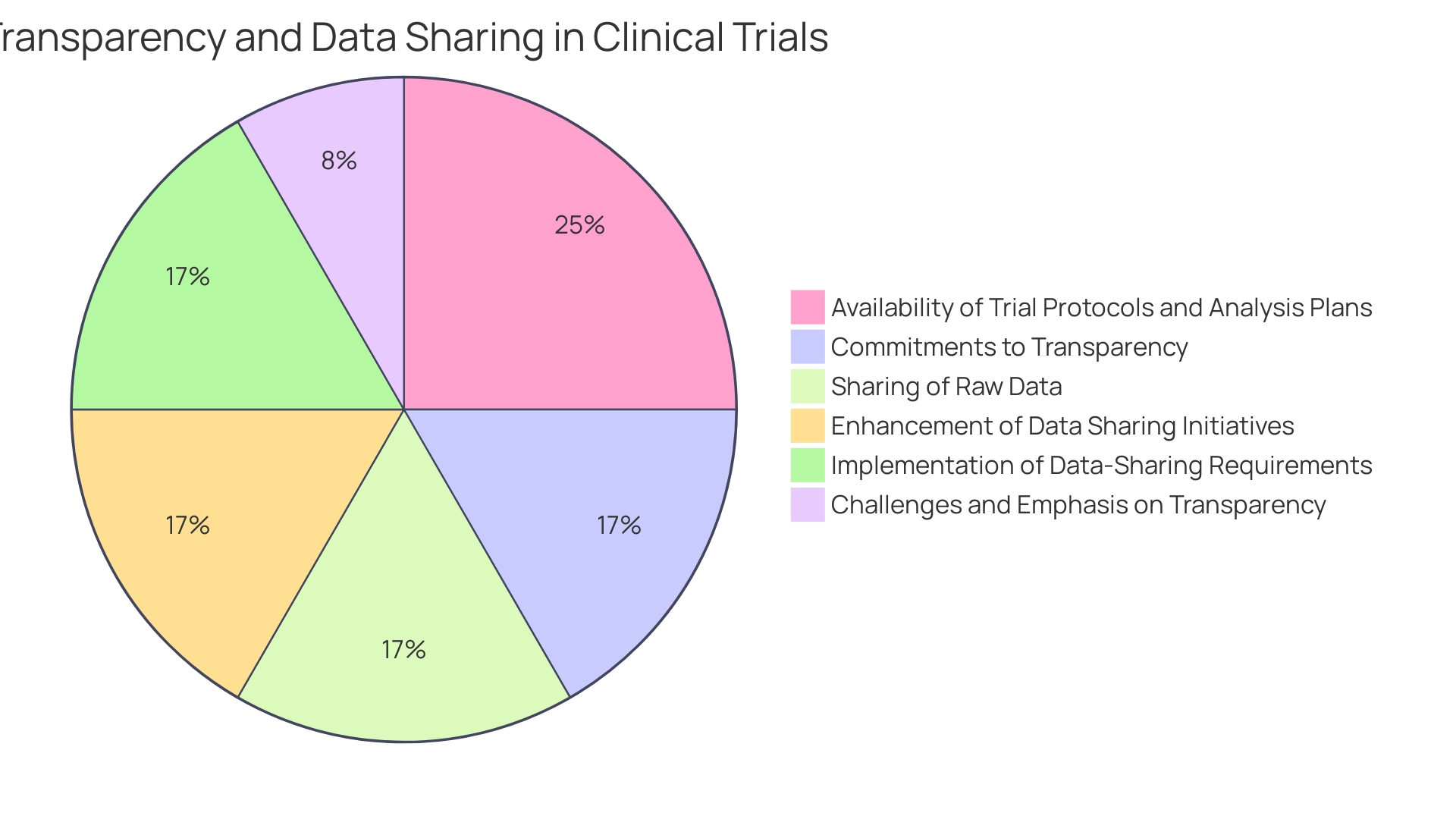
It is well recognized that industry-sponsored trials have become predominant in clinical research, particularly clinical trials. Yet, commitments to transparency vary widely among companies, and there is often insufficient public access to trial protocols and statistical analysis plans pre-trial—a situation that hinders the reproducibility and transparency of research. Despite initiatives to enhance data sharing, such as journal publication policies endorsed by the International Committee of Medical Journal Editors, access to raw data from trials remains limited. This lack of systematic data sharing is a significant concern; it is likely that many trials are analyzed solely by industry statisticians, with raw data not disseminated to wider research communities, including trialists themselves.
Amidst discussions about clinical trial transparency, the FDA has addressed a petition for greater disclosure with a mixed response, highlighting the complexity of balancing transparency with the protection of proprietary information. The FDA favors voluntary compliance over stringent enforcement actions, such as fines, but retains discretionary power to penalize for non-compliance. They have taken steps toward greater accountability, including the creation of a dashboard for notices sent to entities that fail to register clinical trials or report study results.
Ultimately, the importance of cross-referencing data from multiple sources is clear. Discrepancies in adverse event reporting between ClinicalTrials. Gov and published articles could lead to underreporting or selective reporting, an issue that demands attention for the integrity of clinical research and the safety of patients. The collective efforts of regulators, the pharmaceutical industry, and the public are essential in fostering a culture of transparency and trust in clinical trials.
Case Study: Underreporting of Adverse Events in Published Articles
In a comprehensive analysis, we uncover the consequences of insufficiently reported serious adverse events (SAEs) within clinical study outcomes, particularly highlighted by a case where emergency room (ER) visits post-abortion were not properly documented in published articles. The pivotal case in question involved a research paper by Studnicki et al., which sought to characterize ER visits following medication and procedural abortions based on Medicaid claims over a 16-year span. This paper faced retraction due to concerns over research integrity, emphasizing the vital need for robust adherence to the principles of honesty, accuracy, efficiency, and objectivity in healthcare research—principles that are critical when the stakes involve public health policy and access to essential medications like mifepristone.
The incident is a stark reminder of the 'file-drawer problem' identified by psychologist Robert Rosenthal in 1979, describing the tendency to overlook negative or non-supportive results. Over time, this has led to a troubling trend: a study in 2012 found a 22% increase in positive conclusions in published papers between 1990 and 2007. By 2007, an overwhelming 85% of papers reported positive outcomes, overshadowing the critical insights that negative results offer. A 2022 survey highlighted this issue further, revealing that while a significant portion of researchers had negative findings they deemed worth publishing, only a small fraction found opportunities to share them.
This underreporting is not without consequence. It can obscure the true risks associated with clinical trial participation, which often includes a rigorous and taxing regimen of tests and potential exposure to side effects, as noted by clinical and policy researchers like Gamertsfelder and Osipenko. Participants, many of whom are managing complex health conditions, endure these burdens in the hope of advancing treatment options for future generations, despite the immediate personal toll.
Recent news underscores the importance of vigilance in research publication. The Maxima Center's retraction of a study on the impact of COVID measures on child cancer mortality rates after an inappropriate shift in focus from their field of expertise serves as a cautionary tale. It illustrates the necessity for researchers to maintain a keen awareness of their study's scope and integrity, ensuring that findings are reported with the utmost precision and trustworthiness.
Ultimately, the integrity of clinical research is paramount, not only for the advancement of science but also for the trust it commands from the public, policymakers, and the healthcare community at large. Each case of underreported Sales chips away at the foundation of this trust, highlighting the urgent need for a cultural shift towards greater transparency and accountability in the dissemination of clinical research findings.
Consequences of Selective Reporting: Examples and Implications
The integrity of clinical trial reporting is a cornerstone of medical research, impacting patient safety and public trust. Selective reporting of adverse events can skew data, leading to misinformed decisions regarding treatment options. The Yellow Card Scheme (YCS), established in 1964, exemplifies a system designed to detect adverse drug reactions (ADRs) early. It allows individuals to report suspected ADRs to the Medicines and Healthcare Regulatory Agency (MHRA), providing valuable post-market surveillance data. A survey among reporters highlighted that 81% initiated the report themselves, with 49% learning about the scheme from pharmacies.
Evidence from recent court-ordered disclosures following the authorization of the Pfizer/BioNTech vaccine underscores the importance of transparent reporting. Initial trial results indicated fewer deaths in the vaccination group compared to the placebo group. However, more comprehensive data revealed a closer ratio of deaths between the two groups upon further review. These findings illustrate how the timing and completeness of reporting can influence public perception and trust.
In the realm of urology, a study observed a significant increase in the reporting of harms from 2012 to 2020, indicating progress toward meeting transparency and accuracy standards. Nevertheless, the call for consistent and comprehensive reporting persists across all clinical trials. The Vaccine Adverse Event Reporting System (VAERS), despite being a passive surveillance system, has played a pivotal role in identifying vaccine safety issues. By addressing common myths and demonstrating its effectiveness, VAERS has become a critical component of the national vaccine safety system.
As researchers, the responsibility to report all outcomes of trials, as emphasized by experts in the field, is paramount. This steadfast rule ensures that outcome reporting remains consistent over time, contributing to the reliability of clinical research. With the collective goal of safeguarding patient health and maintaining credibility, the medical community must continue to advocate for and practice unbiased reporting of all clinical trial data.
Role of Clinical Research Associates (CRAs) in Monitoring and Documenting Adverse Events
Clinical Research Associates (Cras) are pivotal in safeguarding participants and maintaining the integrity of clinical trials through meticulous monitoring and documentation of adverse events. Their role encompasses educating patients about the trial, as seen in the Neuro-Oncology Clinic at NIH, managing clinical trial logistics, and providing care. This education and support are vital as patients navigate the complexities and potential burdens of participation, often in the hope of benefiting future generations despite the personal toll it may take on them. As highlighted in a JAMA paper, the traditional separation between clinical trialists and clinicians has led to inefficiencies and limitations in the scope and impact of trials. This underscores the necessity for Cras to work in close collaboration with clinical teams to bridge the gap between research and clinical practice.
CRAs are tasked with ensuring that the investigational plan is followed, a responsibility that is paramount to the protection of participants and the validity of the data collected, as emphasized by the Regulatory Affairs Professionals Society. The recent call to enforce trial reporting requirements further stresses the importance of Cras in maintaining high standards of clinical research. Their vigilant reporting of adverse events contributes significantly to the safety and success of clinical trials, reflecting the dedication of researchers who strive to improve the quality and integrity of clinical research.
Clinical trials, which annually register approximately 40,000 RCTs on ClinicalTrials.gov, are instrumental in determining the safety and efficacy of new treatments. Cras play a key role in the three main stages of these trials, from ensuring the safety of a small group of healthy volunteers in phase one, to evaluating efficacy and safety with a larger participant group in phase two, and ultimately contributing to treatments that can change lives. Their documentation of serious adverse events (SAEs) and other safety data is crucial to the ongoing assessment of a treatment's risk-benefit profile, which is essential for patient safety and the advancement of medical knowledge.
Best Practices for Documenting and Reporting Adverse Events
Ensuring the integrity of clinical trial reporting is a multi-faceted challenge that requires meticulous documentation and transparent communication. Standardized reporting forms are foundational to this process, as they provide a consistent framework for capturing adverse event data. Researchers must also establish clear communication channels to facilitate the swift reporting and management of adverse events. This includes the use of robust data management systems that can handle the complexity and volume of trial data, and help maintain the quality and transparency essential for the advancement of medical knowledge.
The responsibility of managing adverse events in clinical trials is underscored by the experiences of participants, such as those with transthyretin-mediated amyloidosis. The personal toll on these individuals is significant, as they navigate numerous health challenges while contributing to research that may benefit future generations. This highlights the critical nature of accurate and empathetic adverse event reporting.
Moreover, recent developments in the field, such as the controversy over image integrity in a pivotal Alzheimer's disease study, underscore the importance of data accuracy. With image-related problems affecting up to 35% of manuscripts, it is clear that effective practices for checking and managing data are essential to the credibility of clinical research.
In light of these challenges, it is imperative for researchers to employ best practices in documentation and reporting. The Vaccine Adverse Event Reporting System (VAERS) exemplifies a successful model for monitoring safety issues, proving the efficacy of vigilant and systematic data collection. Similarly, the Yellow Card Scheme (YCS) illustrates the value of public reporting in identifying potential harms, with a majority of respondents reporting adverse drug reactions on their own initiative.
As we move forward, the adoption of these best practices in documenting and reporting adverse events will not only improve the quality of clinical trial data but will also contribute to the safety and efficacy of new medical interventions.
Improving Transparency and Accessibility of Adverse Event Data
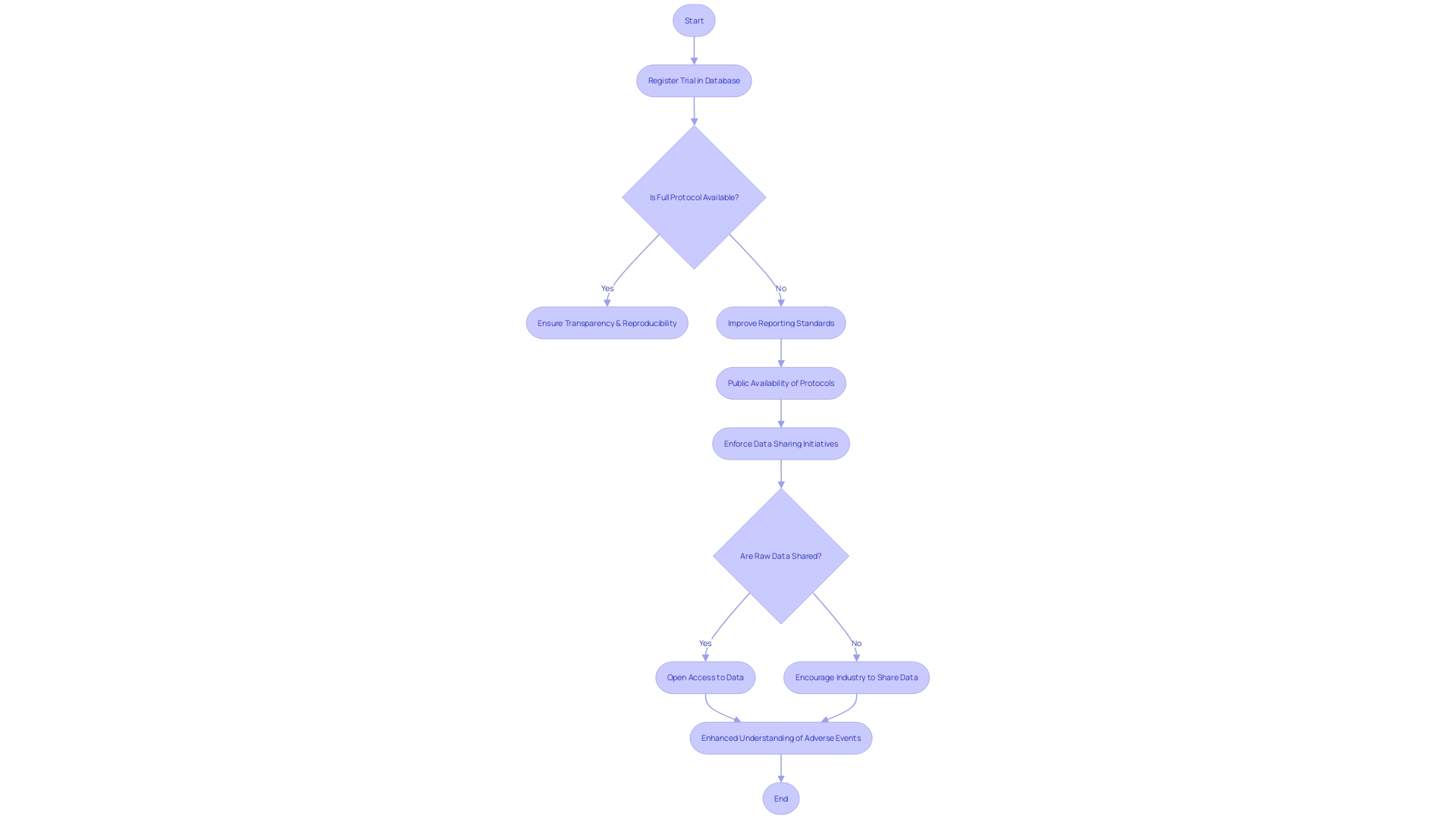
The integrity of clinical research hinges on the transparency and accessibility of adverse event data, which is paramount for patient safety. It is essential to examine the strategies and initiatives that aim to improve this transparency, focusing on the significance of data sharing and the enforcement of reporting standards by regulatory bodies. The benefits of open access platforms for reporting adverse events cannot be overstated, and by creating a culture of transparency and collaboration, researchers can deepen their understanding of serious adverse events and enhance mitigation efforts in clinical trials.
A notable concern is the varying commitment levels from companies regarding trial transparency, often neglecting the public availability of trial protocols and statistical analysis plans. While many trials are registered in databases like ClinicalTrials.gov, such registration may not always include detailed information sufficient for ensuring transparency and reproducibility. Full protocols, analysis plans, and raw trial data should be accessible for genuine transparency. Unfortunately, it appears that most trials sponsored by industry are analyzed solely by in-house statisticians, with raw data seldom shared beyond the immediate research team. Despite numerous initiatives over the last decade to enhance clinical trial data sharing, the adoption of requirements, such as those requested by the International Committee of Medical Journal Editors, remains inadequate for ensuring widespread access to raw data.
ClinicalTrials.gov modernization has taken into account the evolution of trial reporting laws and policies over the past 25 years, striving to display study information effectively and indicate unprovided data by study sponsors and investigators. However, the challenge of maintaining comprehensive and updated records persists, highlighting the need for continual improvement in data element display and explanation.
Moreover, the narrative of patients involved in clinical trials, as shared by researchers like Gamertsfelder and Osipenko, underscores the personal sacrifices made by participants. These individuals, often managing multiple health conditions, participate in trials not for their own benefit but in the hope of sparing future generations from similar suffering. Their experiences serve as a compelling reminder of the ethical imperative for transparency and the impact of clinical research on real lives.
In light of these observations, it is evident that enhancing the transparency and accessibility of serious adverse event data in clinical trials is not just a regulatory issue but a moral one, driving the pursuit of knowledge and the protection of patient welfare.
Conclusion
In conclusion, accurate and comprehensive reporting of Serious Adverse Events (SAEs) in clinical trials is crucial for participant safety and research integrity. A standardized framework for classifying and defining adverse events ensures the identification and capture of SAEs, contributing to the enhancement of intervention safety and patient care.
ClinicalTrials.gov plays a vital role as a platform for transparent reporting of SAEs, providing detailed information for analysis and evaluation. It empowers healthcare professionals, regulators, and the public with accessible data on intervention risks, contributing to informed decision-making.
Discrepancies between adverse event reporting on ClinicalTrials.gov and published articles can undermine research integrity and patient safety. Cross-referencing data from multiple sources is essential to ensure accurate and comprehensive reporting, minimizing the risk of underreporting or selective reporting.
Clinical Research Associates (CRAs) are instrumental in monitoring and documenting adverse events, safeguarding participants, and upholding research standards. Their collaboration with clinical teams bridges the gap between research and practice, ensuring data validity and participant protection.
Best practices for documenting and reporting adverse events include the use of standardized reporting forms, clear communication channels, and robust data management systems. Accurate and empathetic reporting is essential to acknowledge participant burdens and maintain research credibility.
Enhancing transparency and accessibility of adverse event data is crucial for patient safety. Open access platforms and data sharing initiatives promote collaboration and knowledge sharing, deepening our understanding of SAEs and improving intervention safety.
In conclusion, transparent and accurate reporting of adverse events is vital for advancing medical knowledge, protecting patient health, and fostering public trust. The collective efforts of researchers, regulators, and the public contribute to a culture of transparency and accountability in clinical research dissemination, ultimately benefiting patient outcomes and research integrity.




