Introduction
In the realm of pharmaceutical development, the Investigational New Drug (IND) status marks a pivotal milestone, enabling researchers to conduct human clinical trials to test new compounds' safety and efficacy before securing FDA approval for general use. This status is essential for fostering innovative treatments, allowing the administration of experimental drugs to collect valuable data on their pharmacological effects and potential therapeutic advantages. The process entails significant initial investment and extensive development programs, with a small percentage culminating in new drug approvals.
The FDA's Center for Drug Evaluation and Research (CDER) is instrumental in guiding drug developers on study design and data requirements, ensuring new drugs adhere to stringent safety and efficacy standards. The increasing reliance on real-world evidence in clinical development aims to enhance efficiency, reduce costs, and ultimately improve patient outcomes.
What is an Investigational New Drug (IND)?
An Investigational New Drug (IND) signifies an important milestone in pharmaceutical development, enabling researchers to evaluate new compounds for effectiveness and risk in human trials prior to obtaining FDA authorization for widespread use. This status is critical for advancing innovative treatments, as it permits the administration of experimental drugs to gather data on their pharmacological effects and potential therapeutic benefits. The process involves substantial upfront investment and long-term development programs, with only a small fraction leading to new drug approvals. The FDA's Center for Drug Evaluation and Research (CDER) plays a crucial role in guiding drug developers on study design and data requirements, ensuring that new drugs meet stringent standards for effectiveness and well-being. Real-world evidence is increasingly utilized to support medical development, making the process more efficient and cost-effective while aiming to enhance patient outcomes.
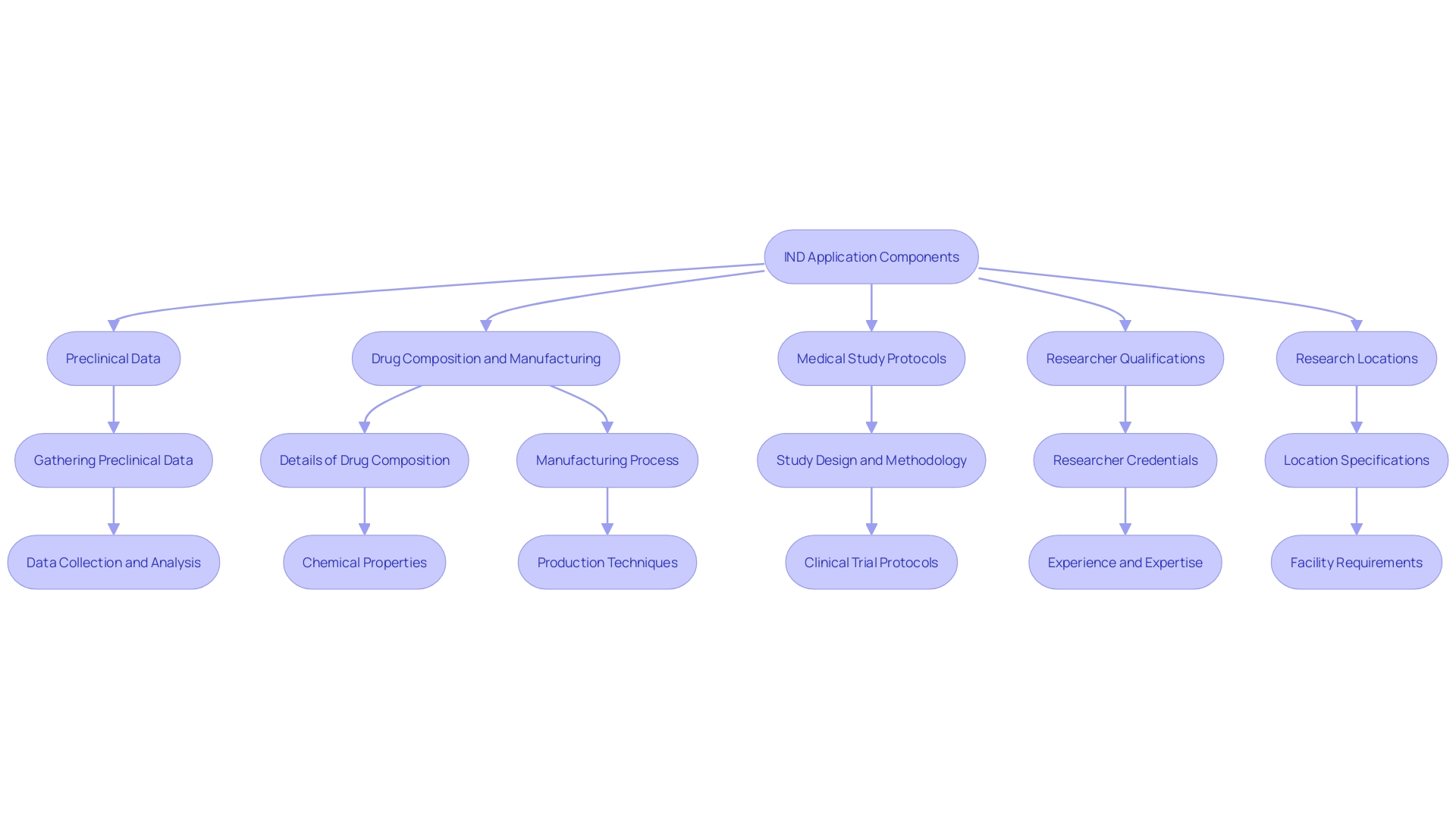
Components of an IND Application
An Investigational New Drug (IND) application is meticulously structured to satisfy the rigorous evaluation criteria set by the FDA. Central to this application are several critical components. Firstly, comprehensive preclinical data must be presented, offering a detailed exploration of the drug's risk profile. This is essential for demonstrating that the drug is safe for initial testing in humans. Additionally, the application must include extensive information about the drug's composition and manufacturing process. This ensures that the drug can be consistently produced and meets quality standards.
The protocols for suggested medical studies are another crucial element of the IND application. These protocols must detail the design and methodology of the tests, aimed at assessing the drug's efficacy and safety in human subjects. The application should also emphasize the qualifications of the researchers who will be carrying out the clinical studies. This encompasses their experience, training, and any previous participation in similar research, ensuring that the evaluations are managed by capable experts.
Furthermore, the IND application must specify the proposed research locations. These locations must be equipped with the necessary infrastructure and resources to carry out the tests effectively. Ensuring that all aspects of the research are well-planned and compliant with regulatory standards is paramount. This includes following protocols on data management, patient well-being, and ethical considerations. By addressing these components comprehensively, the IND application supports a smooth and efficient review process by the FDA.
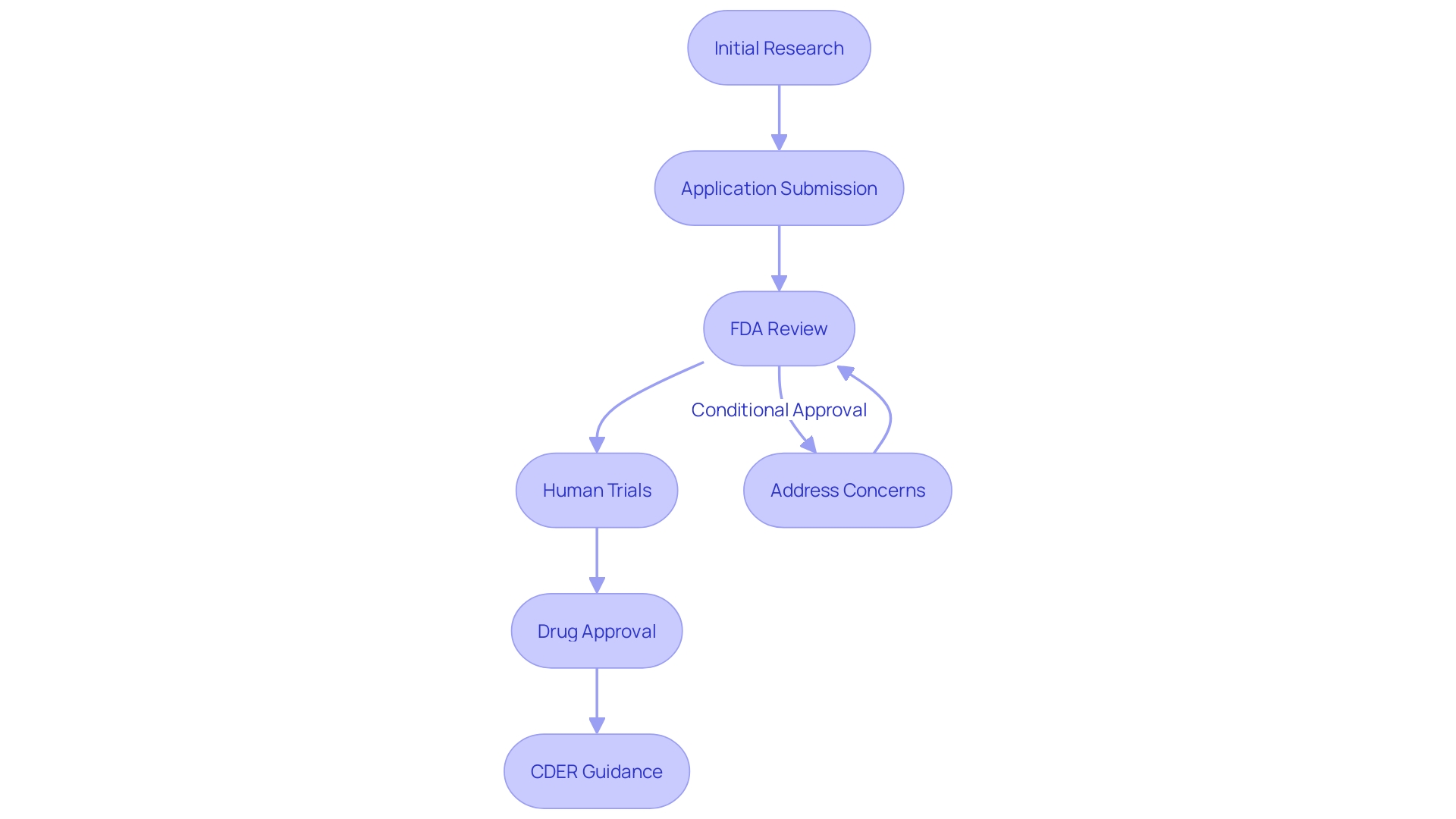
FDA Review of IND Applications
Upon submission of an IND application, the FDA conducts a comprehensive examination to assess the risk and ethical considerations of the proposed research studies. This rigorous review process, which generally spans 30 days, involves the assessment of submitted data and may include requests for additional information or clarifications from the sponsor. If no hold is imposed, the sponsor is authorized to begin the trials as detailed in the application, provided all other regulatory requirements are satisfied. This evaluation is part of the FDA’s wider initiatives to enhance medical research and encourage the creation of evidence required to prove the safety and effectiveness of medical products. The agency also works to harmonize human subject protection regulations with the HHS Common Rule, ensuring that clinical research remains efficient and that participants' rights and welfare are safeguarded. This harmonization aligns with the FDA's final rule, which allows for certain waivers or alterations of informed consent under conditions that pose minimal risk to participants in the study. These measures collectively aim to enhance medical product development while upholding the highest standards of participant protection.
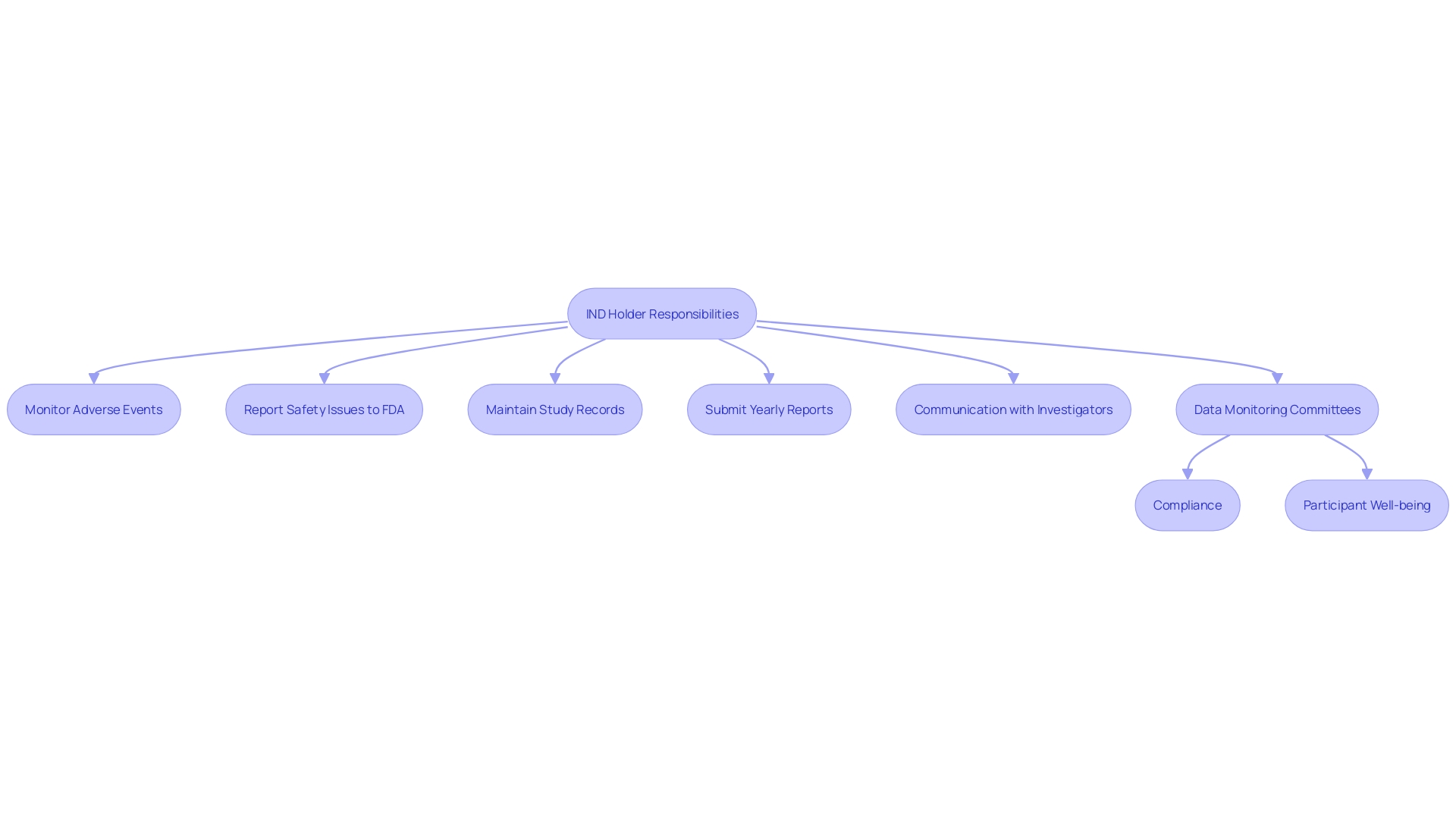
IND Holder Responsibilities
The holder of an IND is responsible for protecting the well-being of study participants and ensuring compliance with regulatory requirements throughout the research. This responsibility encompasses the monitoring of adverse events, the timely reporting of safety issues to the FDA, and the meticulous maintenance of accurate study records. Incorporating Data Monitoring Committees (DMCs) can be instrumental in overseeing these activities, as suggested by recent FDA guidance documents. The IND holder must also submit yearly reports to the FDA, detailing the study's findings and any protocol modifications, thus ensuring sustained transparency and oversight. For example, a program at an institution demonstrated the importance of adherence by revising policies and creating standard operating procedures, which led to a significant reduction in non-compliant studies from 44%. As emphasized in a recent FDA report, clear and consistent communication with investigators is crucial for maintaining compliance and ensuring the integrity of the trial.
Conclusion
The Investigational New Drug (IND) status is a crucial step in pharmaceutical development, enabling human clinical trials that assess the safety and efficacy of new compounds. This status not only fosters innovative treatments but also allows for the collection of vital data on experimental drugs. The IND application requires comprehensive preclinical data, detailed study protocols, and investigator qualifications to ensure compliance with FDA standards.
The FDA conducts a thorough review of IND applications, typically within 30 days, to evaluate safety and ethical considerations. This process may involve requests for additional information from the sponsor, and upon approval, permits the initiation of clinical trials under strict regulatory requirements. The FDA’s focus on harmonizing human subject protection regulations enhances the efficiency of clinical research while safeguarding participants' rights.
The responsibilities of the IND holder are essential for maintaining trial integrity, including monitoring adverse events, timely reporting to the FDA, and submitting annual reports. Effective communication and adherence to compliance are critical for minimizing risks and ensuring successful clinical trials.
In summary, the IND process is vital for advancing new therapies and underscores the importance of rigorous evaluation and regulatory compliance in the development of safe and effective medical products, ultimately aiming to enhance patient outcomes and public health.




