Introduction
Navigating the FDA's regulatory landscape is essential for medical device manufacturers seeking 510(k) clearance. With a comprehensive framework encompassing a range of products, understanding the nuances between 'servicing' and 'remanufacturing' is crucial. The FDA's recent guidance aims to demystify this distinction, ensuring regulatory duties are met.
Voluntary consensus standards and conformity assessments play fundamental roles in maintaining regulatory quality. While the FDA advocates for change control plans to streamline compliance for AI- and ML-enabled devices, resistance to modernization hinders innovation. The FDA's commitment to public health extends to overseeing food, cosmetics, dietary supplements, and tobacco products.
With a plethora of recent guidance releases, the FDA is shaping a future of high safety and efficacy standards for medical devices.
Understanding FDA Regulations and Guidelines
Navigating the FDA's regulatory landscape is vital for medical device manufacturers aiming to secure 510(k) clearance. The FDA's rigorous framework encompasses a broad spectrum of products, each with unique complexities and life cycles. The differentiation between 'servicing' and 'remanufacturing' is particularly nuanced, with the latter carrying significant regulatory implications.
The FDA's final guidance seeks to demystify this distinction, ensuring that entities performing these activities understand their regulatory duties and provide labeling that supports the device's quality, safety, and effectiveness throughout its serviceable life.
Voluntary consensus standards, developed by Standards Development Organizations (SDOs) with adherence to principles of transparency and due process, play a fundamental role in the regulatory quality. These standards, which must be open for stakeholder participation and maintain balanced representation, are part of a robust regulatory structure that includes a thorough conformity assessment as defined by OMB. This assessment is a testament that products, processes, or systems meet the specified requirements.
The FDA's commitment to public health extends beyond medical devices to include the oversight of food, cosmetics, dietary supplements, and tobacco products, ensuring their safety, effectiveness, and security.
Innovation within the regulatory framework is critical, and the FDA has been at the forefront of this, advocating for predetermined change control plans that can streamline compliance for AI- and ML-enabled medical devices. However, there is resistance within the medtech industry to modernize compliance programs. The reliance on manual, slow, yet perceived as safe processes hinders the adoption of more efficient methodologies that could foster innovation while maintaining compliance.
To truly harness technological advancements and expedite market delivery, organizations must embrace these new approaches.
The FDA is proactive in its regulatory role, having issued a plethora of guidance recently to support medtech innovation—surpassing those released in the past two decades. These initiatives underscore the FDA's dedication to not just maintaining but advancing public health protections through clear, conspicuous, and neutral presentations of information, such as drug side effects in direct-to-consumer advertising. With the agency's vigilant oversight and continual guidance releases, it is shaping a future where medical devices are held to the highest standards of safety and efficacy.
What is a 510(k) Submission?
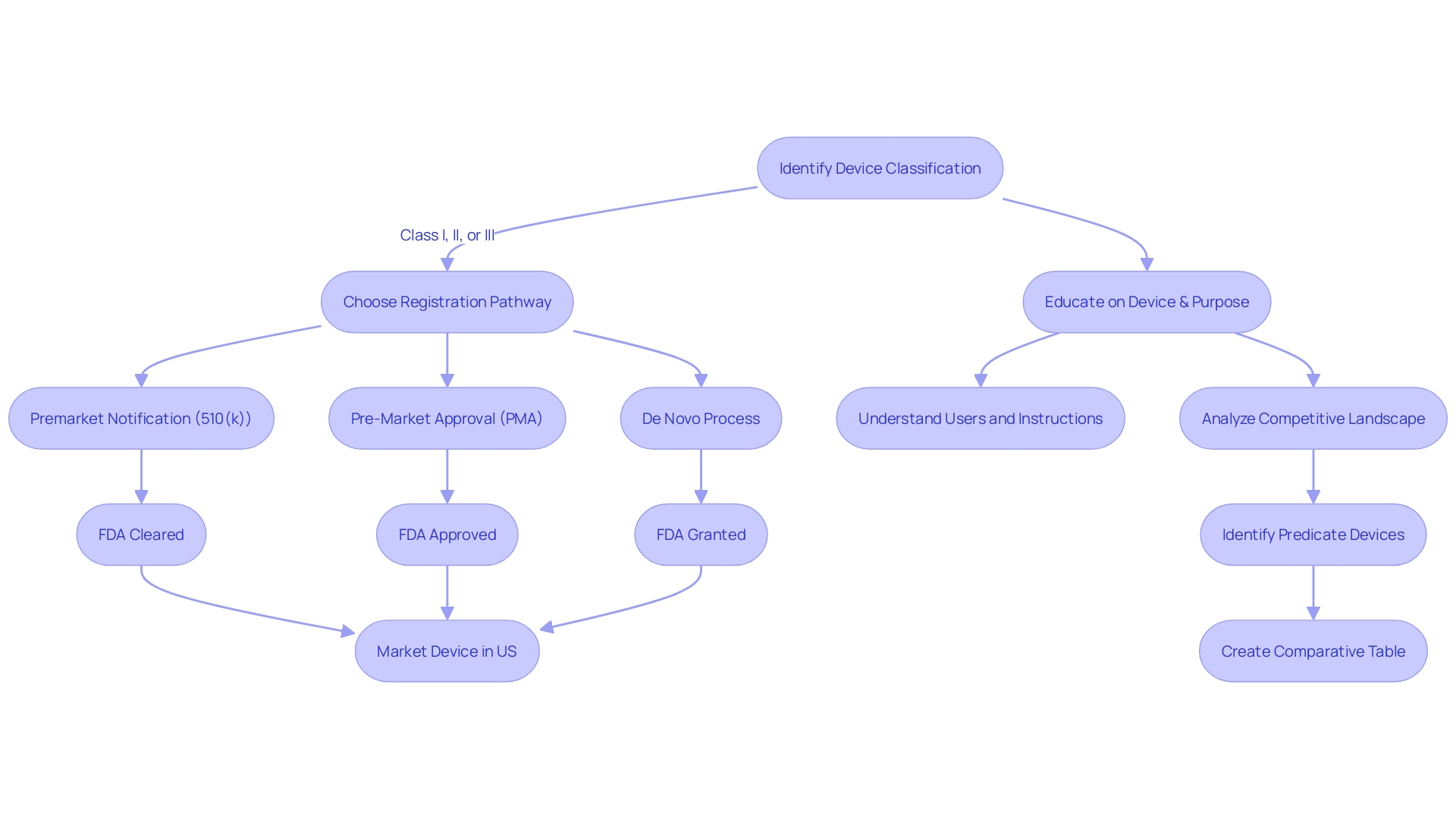
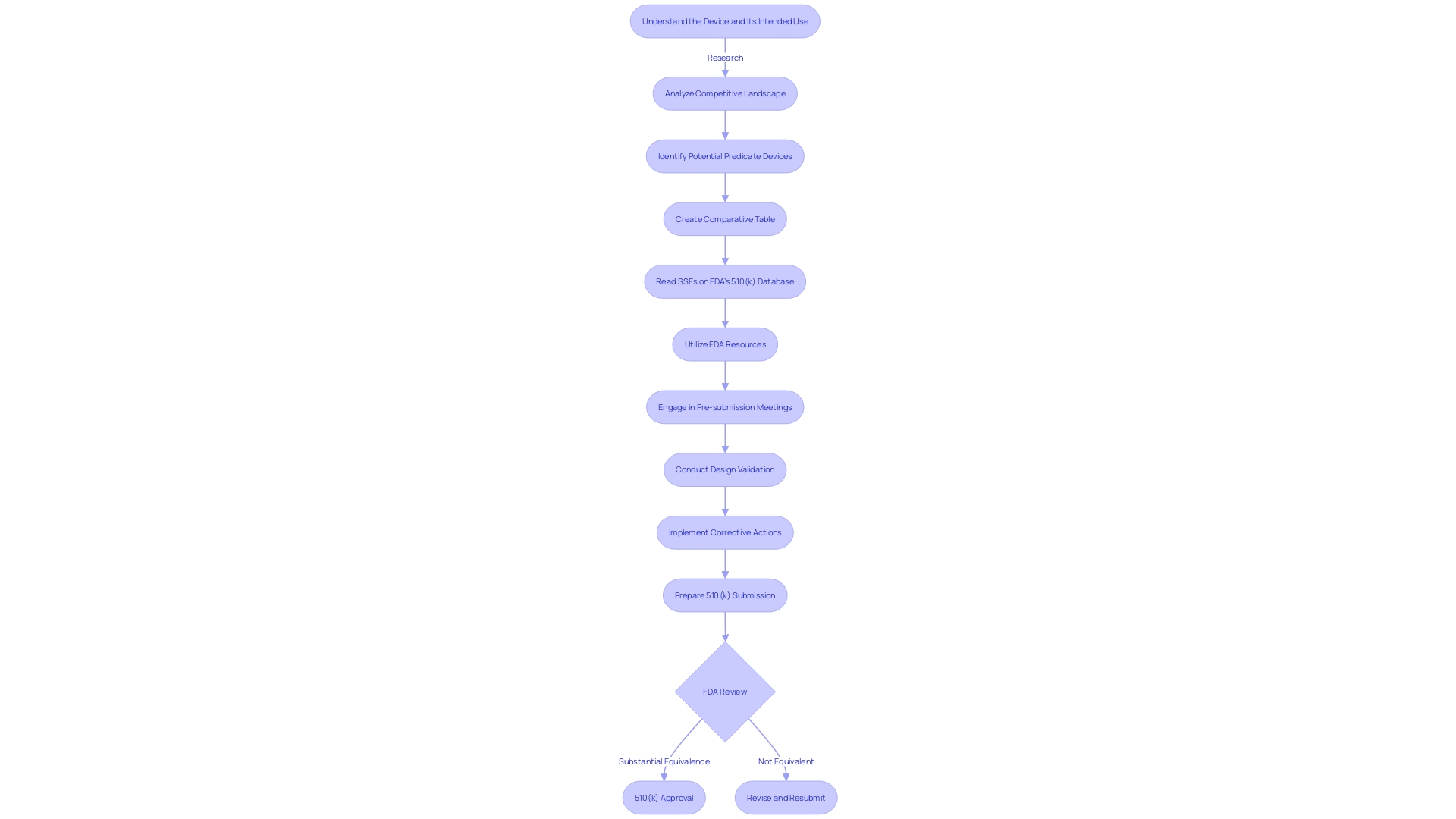
The 510(k) premarket notification is a critical component in the FDA's regulatory oversight of medical devices. Manufacturers use this submission to establish that their device is substantially equivalent to an already legally marketed device, known as the predicate device. Understanding the 510(k) process is not merely a procedural formality; it's a meticulous journey involving in-depth knowledge of both the device in question and its market comparators.
To ensure a successful submission, it is crucial to first deeply understand the subject device, its intended use, and the technological characteristics that define it. This involves examining a wide array of materials, from experiment reports and meeting notes to research literature and clinical studies. Additionally, it's imperative to be familiar with the competitive landscape, identifying competitor devices that could potentially serve as suitable predicates.
A thorough comparative analysis is then developed, often in the form of a table, to delineate the similarities and differences between the subject device and the potential predicate devices. This comparative exercise extends to the review of the Summaries of Safety and Effectiveness (SSEs) available on the FDA's 510(k) database, allowing for a more nuanced evaluation of the device's substantial equivalence.
In the context of regulatory submissions, it is important to recognize the nuances of FDA terminologies such as 'restricted device,' 'classification name,' and 'product code,' which provide a framework for aligning the device with FDA's regulatory classifications. Adding to this, a representative sampling of advertisements and promotional materials should be included to substantiate the claims made for the device.
While the stakes are high and the process is complex, the meticulous preparation and understanding of the 510(k) submission can lead to a significant impact on public health. This is evident as the FDA, through CDER, approves a broad spectrum of medical devices and drugs each year, some of which represent groundbreaking treatments that have never been used in clinical practice. The 510(k) submission is not only about compliance but also about contributing to the advancements in healthcare and therapeutic interventions that benefit the American public.
Types of FDA-Regulated Products and 510(k) Requirements
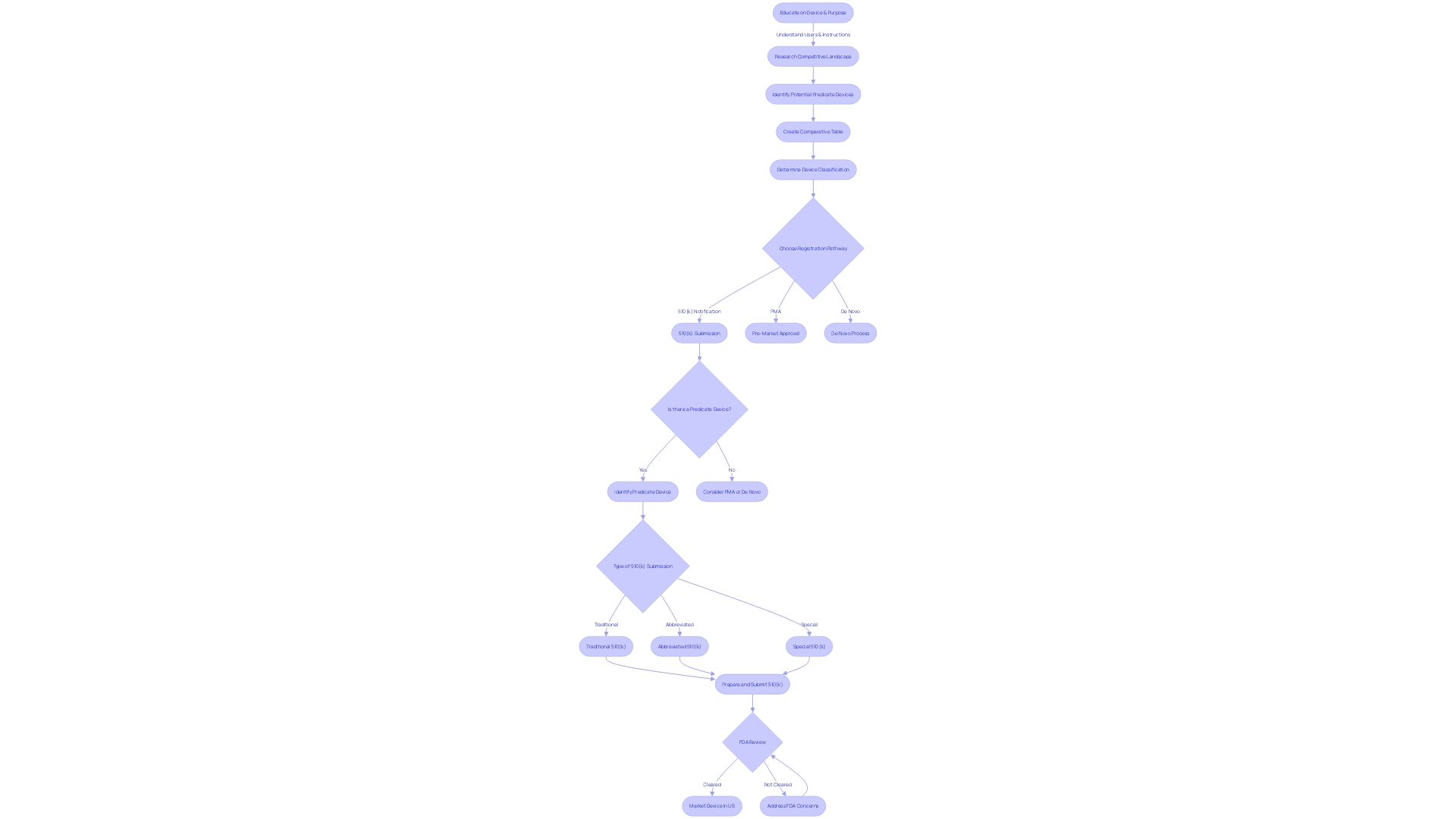
The U.S. Food and Drug Administration (FDA) ensures that medical devices meet stringent safety and effectiveness criteria before they reach the market. The 510(k) submission is a critical step in this process, particularly for devices that fall under Class I, Class II, and Class III categories, each associated with varying levels of risk and regulatory demands.
Class I devices represent the lowest risk category and typically require less regulatory control. Examples include devices like ventricular needles, which are subject to general controls to ensure safety and effectiveness. Class II devices, on the other hand, present moderate risks and, aside from general controls, may require special labeling, mandatory performance standards, and postmarket surveillance to mitigate potential risks to health.
Notably, most neurological devices are classified within this segment. Class III devices, such as implantable pacemakers, represent the highest risk category and are subject to the most stringent regulatory controls, including premarket approval to ensure device safety and effectiveness.
For example, the Impella Connect System, which provides critical ventricular support, demonstrates the complexity of Class II devices with its combination of a hardware module and a software portal that enables remote monitoring of the device's performance. This system, designed to alert healthcare providers of life-threatening conditions through time-critical alarms, exemplifies the type of functionality that requires premarket authorization under section 201(h) of the Federal Food, Drug, and Cosmetic Act.
The case of the Philips Respironics devices, which were recalled after being linked to numerous injuries and deaths, underscores the importance of rigorous premarket testing and ongoing postmarket surveillance. These devices, which had undergone only limited human testing before being marketed, highlight the potential consequences of inadequate device regulation.
With over 1.7 million injuries and 83,000 deaths potentially associated with medical devices over a decade, the FDA actively works to enhance the safety of medical devices through initiatives like active postmarket surveillance systems. These systems continuously analyze data from various sources to detect safety issues that might otherwise remain unreported.
Overall, the FDA's commitment to protecting public health is evident through its rigorous 510(k) approval process, which balances the need for innovation with the imperative of patient safety. Manufacturers must thoroughly understand these requirements to successfully navigate the regulatory landscape and contribute to the advancement of medical technology.
Commodity-Specific Requirements for 510(k) Submissions
The 510(k) medical device approval process is a comprehensive procedure that requires manufacturers to meet specific requirements tailored to the unique characteristics of their medical devices, which vary widely in function and complexity. For instance, the range of devices spans from basic items like eyeglasses and adhesive bandages to technologically sophisticated products such as magnetic resonance imaging (MRI) systems and cardiac pacemakers. With over 10,000 types of medical devices recognized by the World Health Organization, the task of ensuring each device meets FDA standards is no small feat.
Each device must be evaluated for human factors, considering the diverse users, such as clinicians, physicians, dentists, and patients, and their interactions with the device. Instructions for use, including any warnings or precautions, must be clear and comprehensible. Additionally, a thorough understanding of the competitive landscape is essential, requiring manufacturers to review a variety of materials from competitor devices to identify suitable predicate devices with similar intended uses and technological characteristics.
This forms the basis for comparative analyses, which are critical to the 510(k) submission.
Performance testing, biocompatibility assessments, and sterilization validations are core components of the submission, reflecting the range of technologies encompassed by medical devices, including materials science, bioengineering, electronics, and software. These evaluations ensure the devices are safely integrated into healthcare settings without adverse effects on patients.
Moreover, the FDA's classification system stratifies devices into three levels based on the associated patient risk, informing the pathway for registration—be it 510(k) Premarket Notification, Pre-Market Approval (PMA), or the De Novo process. Clear distinctions exist among FDA terms like 'Registered,' 'Cleared,' 'Approved,' and 'Granted,' with each denoting a specific regulatory status that manufacturers must understand to navigate the approval landscape effectively.
Manufacturers must also stay abreast of evolving FDA regulations, such as the recent final rule on direct-to-consumer prescription drug advertisements, which mandates the clear, conspicuous, and neutral presentation of a drug’s major side effects in TV and radio ads. By extension, the medical device industry must ensure labeling requirements are consumer-friendly and easily understandable, reinforcing the importance of comprehensive and accurate submissions for FDA consideration.
In light of the multifaceted nature of medical devices and their regulatory requirements, manufacturers must undertake a diligent and informed approach to their 510(k) submissions, ensuring that all commodity-specific requirements are meticulously addressed to secure FDA clearance.
Data Elements and Values for 510(k) Submissions
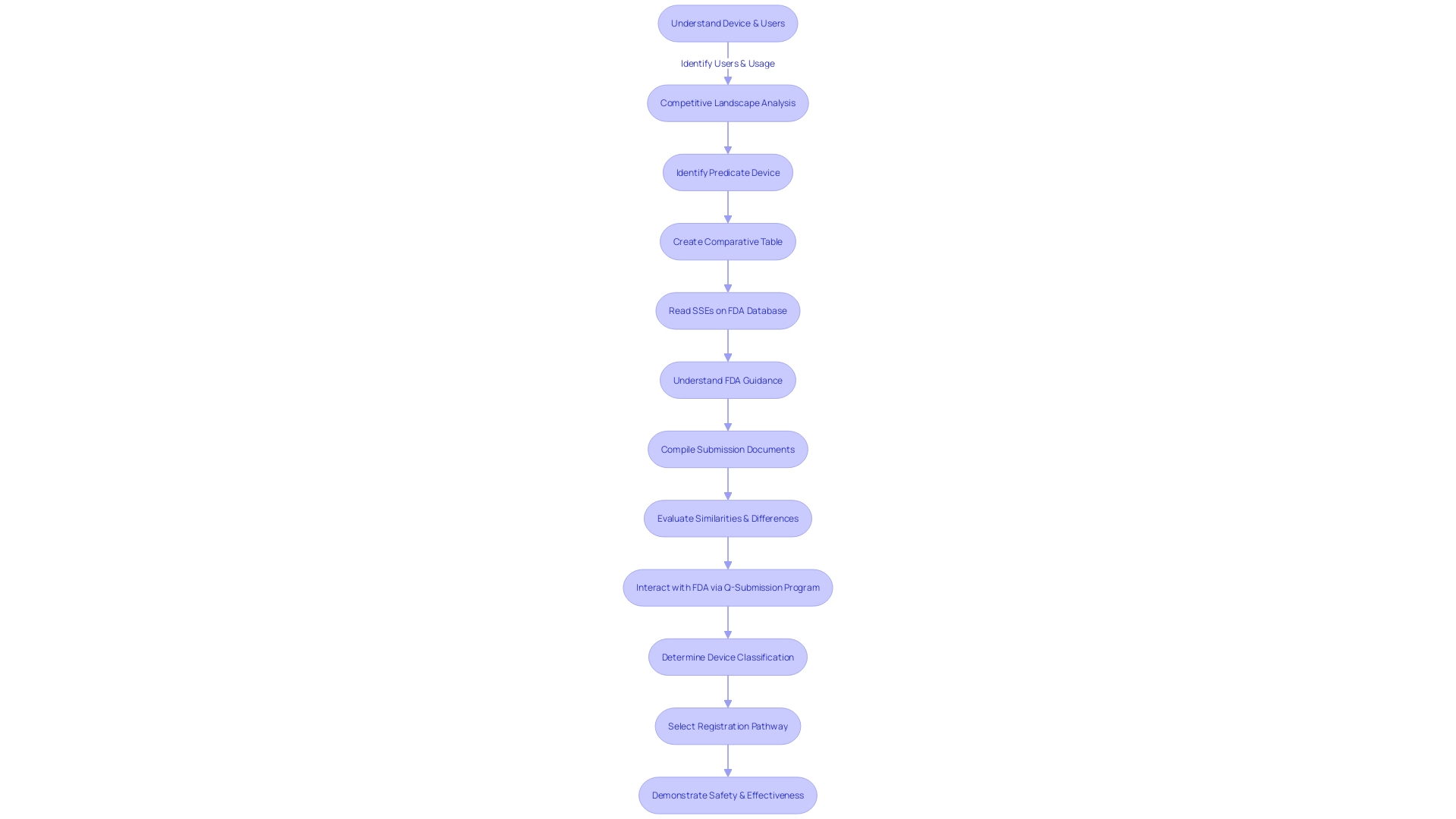
For a 510(k) submission to be successful, it is imperative to provide a comprehensive set of data. This includes an accurate device description, clearly defined intended use, precise indications for use, robust performance data, and relevant clinical data. Each element plays a pivotal role in establishing the substantial equivalence of a medical device to one already on the market.
A crucial initial step involves gaining a thorough understanding of the device, its applications, and the user base, which may range from clinicians and physicians to dentists and patients. Instructions for use must be meticulously detailed, incorporating warnings and precautions. This knowledge is augmented by researching the competitive landscape with the aid of Marketing teams to pinpoint competitor devices that could serve as potential predicates.
By examining research literature, clinical studies, and marketing materials, manufacturers can compile a comparative table outlining the similarities and differences between the new device and existing ones. Reviewing the Summaries of Safety and Effectiveness Data (SSEDs) available in the FDA's 510(k) database is essential in evaluating these aspects.
It is crucial for manufacturers to be well-versed in the FDA's guidance documents and understanding of the submission process to ensure their 510(k) application aligns with the FDA's expectations. The challenge lies in efficiently gathering and compiling the necessary data within the set time frame, ensuring the information supports a decision of substantial equivalence by the FDA.
Moreover, it's important to handle the submission process with confidentiality and care. Any public comments or documents submitted to the FDA should not contain sensitive information, as they become part of the public record. Manufacturers should be prepared to submit confidential information through secure, written submissions as instructed by the FDA.
By diligently following these guidelines and understanding the nuances of the 510(k) process, manufacturers can effectively navigate the regulatory pathway and contribute to the availability of safe and effective medical devices in the market.

Submission Process and Timeline
The process of submitting a 510(k) application is a critical step for manufacturers seeking to bring a new medical device to market. It necessitates meticulous preparation, beginning with a comprehensive understanding of the device and its intended use. Manufacturers must delve into the details of how clinicians, physicians, dentists, and patients will use the device, fully grasping all instructions, warnings, and cautions.
In parallel, a thorough examination of the competitive landscape is essential. This involves researching literature, clinical studies, and marketing materials to identify potential predicate devices that share the same intended use and technological characteristics. Creating a comparative table becomes a foundational task in this phase, aiding in the clear presentation of similarities and differences against existing devices.
Once the groundwork is in place, the focus shifts to the FDA's expectations. The agency's guidance documents provide a roadmap for the submission process, underscoring the importance of adhering to their recommendations. The challenge lies in assembling the required information efficiently to meet the FDA's stringent criteria for a decision of substantial equivalence.
The submission method itself is a carefully structured procedure that includes completing the necessary forms, paying applicable fees, and adhering to submission guidelines. The typical review timeline by the FDA, from acceptance to clearance, is a key consideration for manufacturers to effectively plan and manage their submission strategy.
By integrating insights from successful case studies such as Artos and staying informed about the latest FDA regulations and expectations, manufacturers can navigate the 510(k) submission process with greater confidence, aiming for a smooth transition from application to market entry.

Affirmations of Compliance and Verification
In the 510(k) submission process, manufacturers are required to provide evidence of compliance and conduct thorough verification testing. This vital step ensures that their medical device meets the FDA's rigorous standards for safety and effectiveness. The process includes developing a comprehensive understanding of the device, including its intended use, users, and instructions for use.
Additionally, manufacturers must compare their device against existing ones in the market to identify a suitable predicate device, creating a comparative analysis that delineates similarities and differences.
Manufacturers must adhere to voluntary consensus standards, which are created through a transparent, open, and balanced process by recognized Standards Development Organizations (SDOs). These standards play a crucial role in maintaining regulatory quality and fostering innovation, as they provide guidelines for activities and their results, aiming to achieve a high degree of order within the context they are applied.
Moreover, the FDA emphasizes the importance of public comments in shaping its regulatory actions. However, submitters must carefully avoid including confidential information in their public comments, as these submissions become part of the public record. For comments containing sensitive information, a written/paper submission process is available to protect confidentiality while allowing for stakeholder input.
Understanding the lifecycle of a medical device, from development to post-market assessment, is essential for ensuring public health, as evidenced by the FDA's comprehensive evaluation of vaccines. The agency's commitment to safety and effectiveness is reflected in its ongoing efforts to monitor and regulate medical products throughout their use.
For manufacturers, it is crucial to provide detailed information regarding corrective actions taken in response to compliance issues, as evidenced by the FDA's response to a firm's actions addressing complaint coding and quality data analysis. The response must include outcomes of these actions, which are vital in demonstrating compliance and maintaining the integrity of the regulatory process.

Common Issues and Best Practices in 510(k) Submissions
Achieving a successful 510(k) submission requires not only a thorough understanding of the device itself but also a comprehensive comparative analysis with existing, similar devices on the market. The preparation phase should begin with a deep dive into understanding the users of the device, its intended uses, and any associated warnings or cautions. Collaborating with the marketing team is crucial to understand the competitive landscape, which involves researching existing literature, clinical studies, and competitor marketing materials.
These efforts facilitate the identification of a suitable predicate device that shares the same intended use and technological characteristics.
Creating a detailed comparative table is an essential step, which should include an examination of the Summaries of Safety and Effectiveness Data (Seeds) from the FDA's 510(k) database. This analysis will highlight the similarities and differences between your device and the chosen predicate, aiding in demonstrating substantial equivalence. Moreover, it is crucial to anticipate and plan for the complexities of mass production when working with contract manufacturers, acknowledging that replicating a successful prototype in large quantities is a distinct and challenging phase of development.
Manufacturers often face obstacles such as insufficient device descriptions, incomplete testing data, and unclear submissions. To avoid these pitfalls, it is advised to adhere to the FDA's guidance documents and familiarize oneself with the 510(k) submission process detailed on the FDA's website. By doing so, manufacturers can ensure that their submission contains all the necessary information, presented in a manner that meets the FDA's expectations for a determination of substantial equivalence.
In addition to these practical steps, staying informed about the latest innovations and approaches in health technology, such as new user interfaces for medical devices, can provide valuable insights into the evolving regulatory landscape.
Resources and Tools for 510(k) Submissions
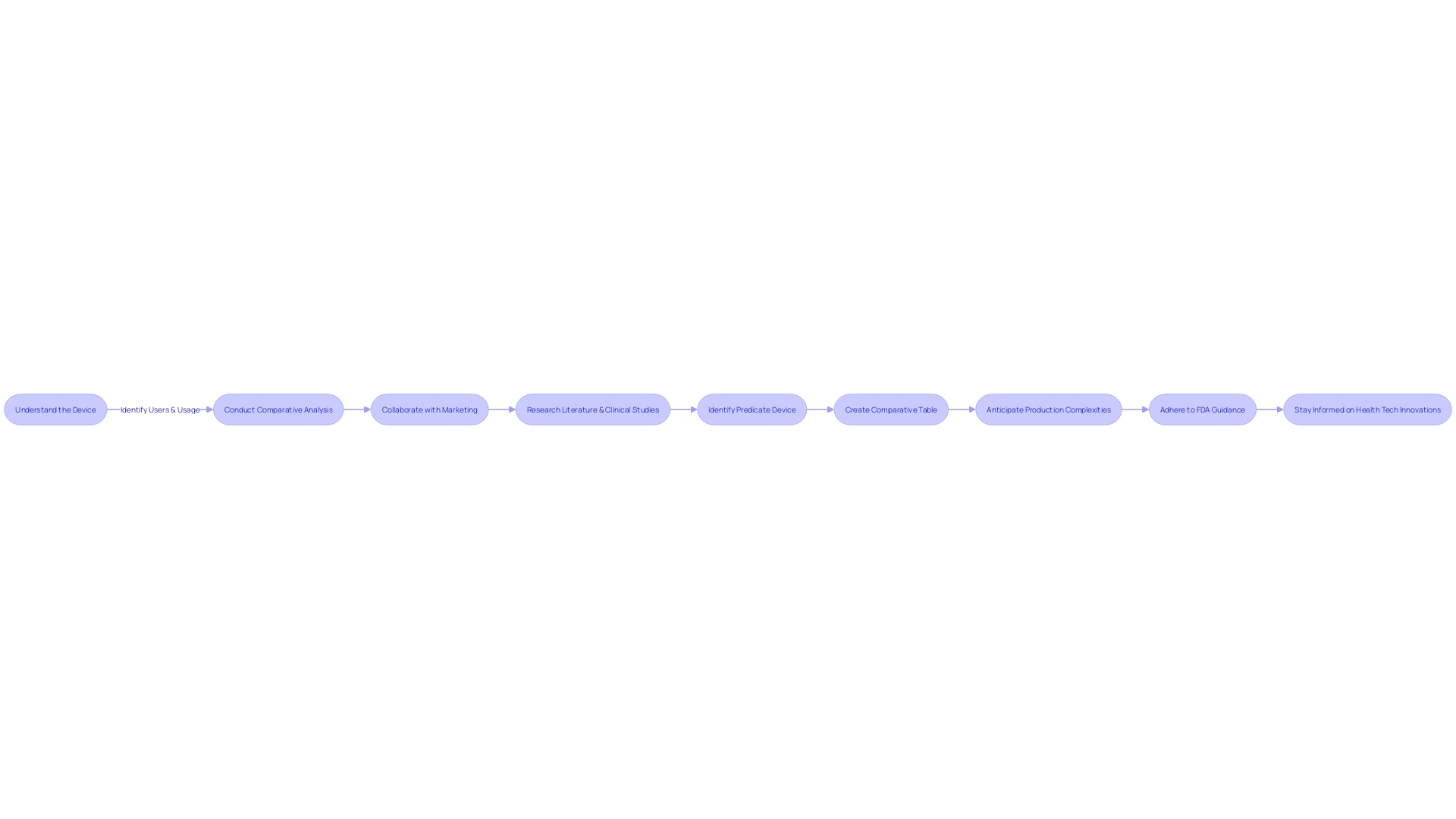
Navigating the 510(k) medical device approval process requires a robust understanding of the FDA's regulatory framework. For manufacturers, comprehending this process is vital to ensure a smooth path to market for their devices. A critical step is to become well-informed about the device in question, including its intended use, users (such as clinicians and patients), and any instructions for use, accompanied by warnings and cautions.
This foundational knowledge should be complemented by a thorough analysis of the competitive landscape, identifying potential predicate devices with comparable intended uses and technological characteristics. This analysis can be supported by a variety of resources offered by the FDA, including guidance documents, online databases, and pre-submission meetings.
These FDA-provided tools serve as a compass for manufacturers, aiding them in understanding the nuances of the 510(k) process. For instance, by delving into the Summaries of Safety and Effectiveness Data (Sets) available on the FDA's 510(k) database, manufacturers can assess similarities and differences with predicate devices, an essential component of the submission. Additionally, manufacturers are encouraged to engage in pre-submission meetings with the FDA.
These interactions can offer invaluable insights and guidance, potentially expediting the approval process and ensuring that submissions meet all necessary regulatory requirements.
Moreover, in light of recent scrutiny, such as the 2018 documentary 'The Bleeding Edge', which revealed that clinical trials are not mandatory for many devices under the 510(k) process, manufacturers must be diligent in their design validation procedures, including comprehensive risk analysis. The documentary underscored the importance of rigorous safety evaluations, as some fast-tracked devices have resulted in patient injuries.
To further strengthen their submissions, manufacturers should consider systemic corrective actions, like those taken by a firm in response to a complaint coding issue. This involved a retrospective review of complaints, procedure revisions, and staff training, alongside an assessment (CAPA 2023-042) to address historical complaints and determine the need for additional remediation actions.
Ultimately, by utilizing the wealth of resources provided by the FDA and ensuring adherence to regulatory standards, manufacturers can navigate the 510(k) approval process with greater confidence and precision, contributing to the safety and effectiveness of medical devices in the market.
Conclusion
In conclusion, navigating the FDA's regulatory landscape is crucial for medical device manufacturers seeking 510(k) clearance. The FDA's recent guidance aims to demystify the distinction between 'servicing' and 'remanufacturing' and ensure regulatory duties are met.
Voluntary consensus standards and change control plans for AI- and ML-enabled devices play a fundamental role in maintaining regulatory quality. Resistance to modernization hinders innovation, but the FDA is committed to shaping a future of high safety and efficacy standards for medical devices.
Successfully navigating the 510(k) submission process requires understanding FDA terminologies, complying with regulations, and providing comprehensive data. Adhering to the FDA's guidance documents, ensuring consumer-friendly labeling, and addressing compliance issues are crucial for a successful submission.
Common issues in 510(k) submissions include insufficient device descriptions and incomplete testing data. Adhering to FDA guidance, familiarizing oneself with the submission process, and staying informed about health technology innovations can help manufacturers navigate successfully.
By utilizing FDA resources, such as guidance documents and pre-submission meetings, manufacturers can navigate the regulatory landscape with confidence. Design validation and comprehensive risk analyses are important for device safety. Following best practices and utilizing available resources contribute to the availability of safe and effective medical devices in the market.
The FDA's commitment to protecting public health is evident through its rigorous 510(k) approval process, balancing innovation with patient safety. Navigating the 510(k) medical device approval process requires a robust understanding of the FDA's regulatory framework. Manufacturers play a crucial role in advancing medical technology while ensuring compliance with FDA standards.




