Introduction
Clinical trials play a crucial role in evaluating the effectiveness and safety of new interventions. One important aspect of these trials is the use of active control groups, which receive established treatments that serve as benchmarks for comparison. However, the selection and design of these control groups can present challenges and ethical considerations.
In this article, we will explore the concept of active control in clinical trials and its scientific merit, as well as discuss the types of control groups used and the methodological considerations involved. We will also examine real-life case studies that highlight the successful use of active control groups. By delving into these topics, we aim to provide accurate and detailed information to our readers, ensuring that they have a comprehensive understanding of the importance of active control groups in clinical research.
What is Active Control in Clinical Trials?
In clinical trials, an active comparison category is crucial as it receives a known effective treatment, which serves as a benchmark against the experimental cohort's new intervention. This approach was apparent in a study where researchers imaged individuals discharged after an acute infectious disease hospitalization. They compared these images with those from a younger, healthier comparison set, not admitted for similar illnesses. Although statistical adjustments were made, the notable distinctions in patient demographics could not be sufficiently handled, which gives rise to doubts regarding the reliability of the comparison due to the inequality in the number of individuals, with 259 patients in the treatment category compared to 52 in the experimental category. This highlights the significance of appropriately matched command cohorts in clinical experiments to guarantee significant and trustworthy outcomes. In the DAPA-MI study, researchers utilized a registry-based randomized controlled assessment (R-RCT) design, combining real-world data with randomized evaluation elements to evaluate a marketed product's efficacy with a known safety profile. Despite initial expectations, the number of primary outcomes was significantly lower than anticipated, highlighting the unpredictable nature of clinical research and the need for adaptable study designs.
Ethical Considerations for Using Active Control Groups
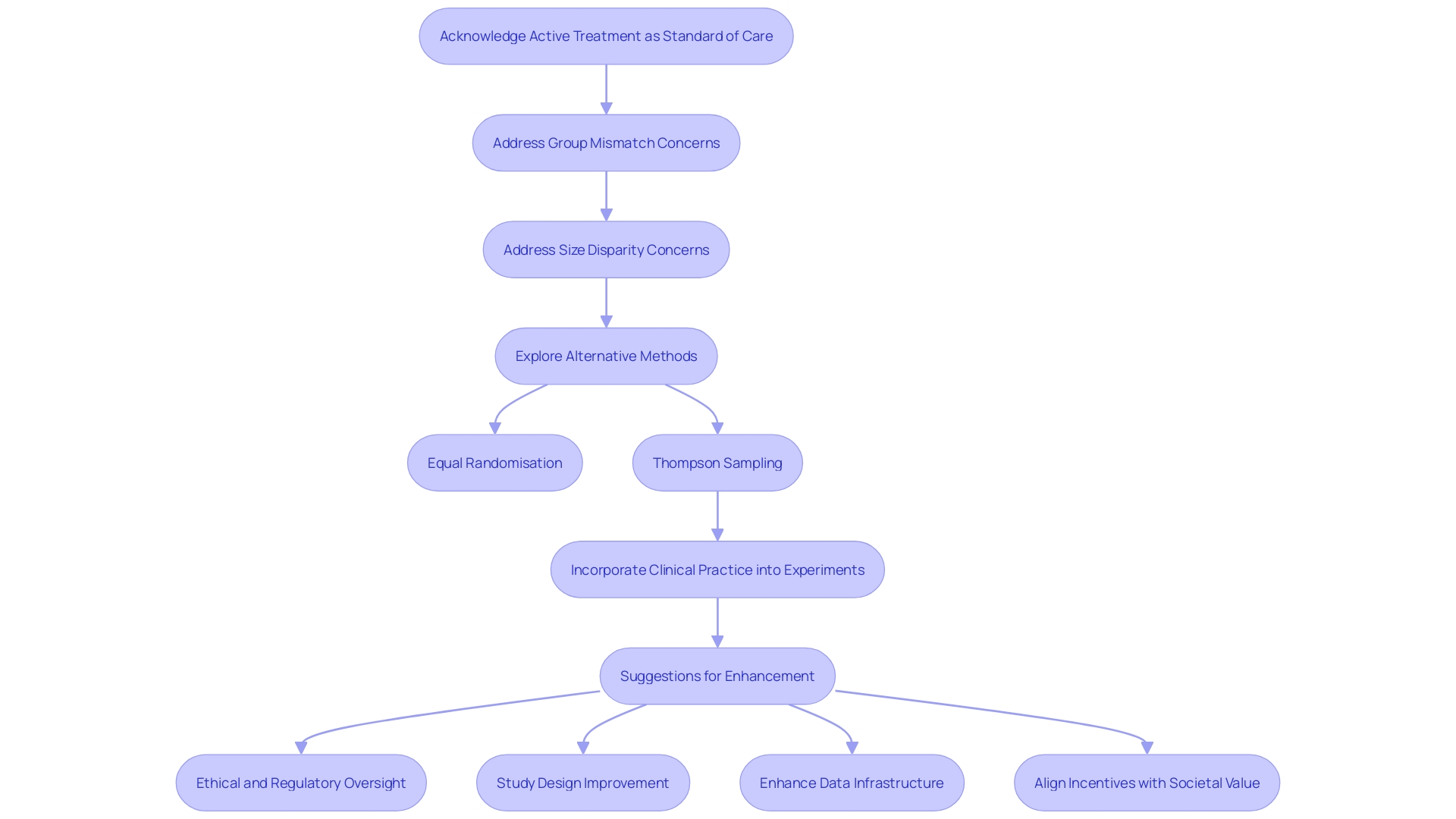
Ethical considerations in clinical trials with active comparison sets are of utmost importance to guarantee the integrity and societal value of the research. The active treatment must be acknowledged as the standard of care, and this is emphasized by the recent case where researchers compared the imaging of patients discharged after acute infectious diseases to a comparison set that was significantly younger and healthier. This mismatch, coupled with the significant disparity in group sizes - 259 in the active group and only 52 in the control - raises concerns about the validity of any statistical adjustments made. In clinical experiments, it is crucial to not only offer ethical treatment but also to uphold the validity and reliability of the outcomes. Methods such as Equal Randomisation and Thompson Sampling offer alternatives, but each comes with its own set of considerations. The former does not always maximize statistical power, while the latter aligns treatment probability to the likelihood of it being the most effective. Moreover, recent conversations at the JAMA Summit and publications in JAMA have emphasized the necessity for clinical experiments to more effectively incorporate with clinical practice, emphasizing that the separation of experimenters and clinicians results in inefficiencies and constraints in experiment impact. Suggestions for enhancement involve ethical and regulatory oversight, study design, data infrastructure, and incentive alignment across clinical examination and health care delivery systems. This emphasizes the necessity for experiments to tackle the appropriate inquiries and integrate into treatment in manners that correspond to significant outcomes, guaranteeing that active comparison sets are efficiently employed within the wider framework of healthcare and scientific investigation.
Scientific Merit of Active Control Groups
Using active control units in clinical trials is crucial to assessing the effectiveness of new treatments. By meticulously comparing outcomes of individuals in the experimental category and a similar set receiving existing standard treatments, researchers can discern the real benefits of the new intervention. For example, a study comparing imaging abnormalities in individuals recently discharged from the hospital with an infectious disease to a cohort of younger, healthier peopledespite efforts to make statistical adjustmentsâemphasized the difficulties in guaranteeing comparability between the two sets. Furthermore, the significant disparity in the sizes of the sets, with 259 individuals in the treatment arm versus only 52 in the control, further complicates the interpretation validity of the results. This underscores the necessity of rigorous statistical analysis and the collaboration with expert statisticians in clinical research, to uphold the integrity and reliability of the study outcomes. Recent advancements in technology, such as electrical stimulation for spinal cord injuries, demonstrate the potential of clinical experiments to transition from research to real-world applications, paving the way for innovations that could substantially improve patient care.
Types of Control Groups in Clinical Trials
Control sets are essential to the design of clinical trials, serving as benchmarks to determine the efficacy and safety of new interventions. Active comparison categories, receiving established effective treatments, contrast with placebo categories, often administered sugar pills, and non-intervention categories, which receive no intervention at all. The use of active management is particularly enlightening when comparing new treatments to standard care, but it's crucial that these comparisons are fair and balanced. An examination, analyzing imaging abnormalities in post-hospitalization individuals, highlights the drawbacks of mismatched comparison sets; 259 individuals in the active category were compared to a considerably smaller, healthier comparison set of 52, raising concerns about the accuracy of the findings due to the stark contrast in individual profiles. Such disparities highlight the need for properly designed active comparison cohorts that are similar in size and health condition to the treatment cohort, guaranteeing that findings are truly ascribed to the treatment rather than unrelated factors. This understanding is critical not only for researchers but also for medical specialists, device industry executives, and informed patients who rely on robust clinical data to make healthcare decisions.
Active Control vs. Placebo Control: Methodological Considerations
Deciding whether to use an active comparison or a placebo in a clinical trial is a crucial choice that depends on the research goals and ethical obligations. Active comparison categories allow for an evaluation of a new intervention versus a well-established therapy, highlighting the relative effectiveness and security. On the other hand, placebo comparison sets are essential in distinguishing the genuine curative impact of a novel intervention, eradicating the impact of placebo effects.
For example, a research involving individuals recently released from hospitalization due to acute infectious disease utilized imaging to compare these individuals to a younger, healthier comparison set rather than to individuals with a similar length of hospital stay for various illnesses. This method, even with statistical efforts to account for disparities, could not alleviate the substantial distinctions between the sets, which consisted of 259 patients in the active branch versus only 52 in the comparison branch. Such imbalances underscore the necessity for careful control selection to ensure reliable results.
Moreover, a meta-analysis of more than 2,700 studies on transcranial magnetic stimulation for managing depression, which narrowed down to 52 experiments with high-quality clinical study data, exhibited the complexities of placebo effects. About half of the 4,500 participants received placebo treatments involving non-functional or misdirected hardware, while the remainder received various transcranial magnetic stimulation techniques. The findings pointed out that while placebo participants reported improvements, the actual treatments generally produced more pronounced effects.
These examples demonstrate the necessity of choosing suitable comparison groups in clinical research, which is reiterated by specialists who stress that the sample population in an experiment must be a representative subset of the eligible patient population. Inferential statistics are then used to project potential outcomes within the entire population that could receive the intervention, understanding that there will always be a numerical difference in baseline characteristics due to randomization. Such methodological rigor is essential to uphold the integrity of clinical research findings.
Active Control Trials: Design and Interpretation Challenges
Developing effective active comparison studies requires a careful strategy to guarantee the choice of a suitable and effective active treatment. It's equally important to have an experiment that's sufficiently powered to detect any differences between the experimental intervention and the active comparison. An outstanding illustration of the intricacies engaged in such experiments is apparent in a study where imaging of individuals released after an acute infectious disease was contrasted to a significantly younger and healthier comparison group. Despite statistical adjustments, the disparities in health status between the 259 patients in the active arm and the 52 in the control arm were too vast to be accounted for, highlighting the challenges in maintaining experiment integrity.
Additionally, experiments must consider confounding factors and potential biases that could distort results. An analysis published in BMJ Global Health illustrates this point, demonstrating how biases like human and mosquito movement can be mitigated, while transmission coupling in dengue virus transmission studies requires complex mathematical modeling beyond traditional clinical examination analysis.
Randomization plays a pivotal role in mitigating these biases. However, it's a misconception that Equal Randomisation (ER)—assigning half the patients to one treatment and half to another—maximizes statistical power. In fact, methods like Thompson Sampling (TS), where treatment assignment is proportional to the current evidence of treatment efficacy, may provide more power. This emphasizes the significance of carefully selecting a randomization approach to most effectively match the objectives of the experiment.
The understanding of experiment outcomes must be done with care to ensure medical decision-making is informed by robust evidence. Trials should aim to maximize treatment success while being cost-effective and informative, a sentiment echoed by researchers and funders alike who strive to navigate the delicate balance between learning and treatment optimization in clinical research.
Case Studies: Successful Use of Active Control Groups
Active comparison categories are a crucial element in clinical trials, acting as a reference point against which new interventions can be evaluated. An impressive illustration comes from a study where researchers employed imaging to evaluate individuals discharged after an acute infectious disease hospitalization. This assemblage of individuals was juxtaposed with a notably younger and healthier reference population, bypassing the typical comparison with individuals hospitalized for other ailments. In spite of the author's attempts at statistical adjustments, the striking difference in demographics between the active arm, comprised of 259 individuals, and the compare branch, with only 52, presented a noteworthy obstacle in formulating dependable conclusions.
This situation highlights the significance of choosing suitable comparison sets to guarantee the credibility of clinical experiments. Such decisions can deeply influence the perceived effectiveness and safety of new treatments. It also emphasizes the potential pitfalls when control groups do not align closely with the population under study, potentially leading to skewed results and misinterpretations regarding organ damage and other indirect effects of diseases.
Reflecting on the broader implications of clinical study design, the innovative DAPA-MI study utilized a registry-based randomized controlled experiment (R-RCE) to merge real-world data with the rigor of randomized trials. Their approach aimed to maximize patient enrollment, maintain cost-efficiency, and establish causal relationships. This trial's evolution also illustrates the dynamic nature of clinical research, where endpoints and methodologies may adapt to emerging data and trends.
In the context of drug development, the FDA's Center for Drug Evaluation and Research (CDER) emphasizes the necessity of meticulous study design and comprehensive data collection. Each year, CDER's approval of a diverse array of new drugs and biological products is predicated on a profound understanding of the science behind these innovations. The rigorous assessment of such products ensures that they meet the high standards required to offer new treatment options and improve public health outcomes.
Conclusion
In conclusion, active control groups are crucial in clinical trials for evaluating new interventions. Ethical considerations and well-matched control groups ensure the integrity and validity of research. By comparing outcomes with established treatments, active control groups provide meaningful and credible results.
The choice between active control and placebo control depends on research objectives and ethics. Meticulous trial design and interpretation are essential for reliable results and informed decision-making. Successful case studies highlight the importance of appropriate control group selection and adaptive methodologies.
In drug development, comprehensive study design and data collection are vital for regulatory approval and improving public health outcomes. Overall, active control groups are indispensable in advancing medical knowledge and enhancing patient care.
Learn more about our adaptive trial design and how it can enhance the validity of your research.




