Introduction
Form FDA 1572 is a critical document that establishes a formal agreement between clinical investigators and study sponsors in the realm of clinical research. This article provides a comprehensive overview of the purpose and regulatory requirements of Form FDA 1572, emphasizing the importance of adherence to ethical conduct and integrity in clinical trials. The article explores the information that must be provided on the form, the individuals responsible for completing it, and when it should be completed and signed.
It also highlights the submission and updating process of Form FDA 1572 and delves into exemptions and special cases where the form may not be mandatory. Additionally, the article discusses common mistakes and best practices in filling out the form, underlining the significance of accurate completion and meticulous record-keeping. By providing accurate and detailed information, this article aims to equip clinical investigators and stakeholders with the knowledge necessary to ensure compliance and uphold the highest standards of participant safety and treatment outcomes in clinical research.
Purpose and Regulatory Requirements of Form FDA 1572
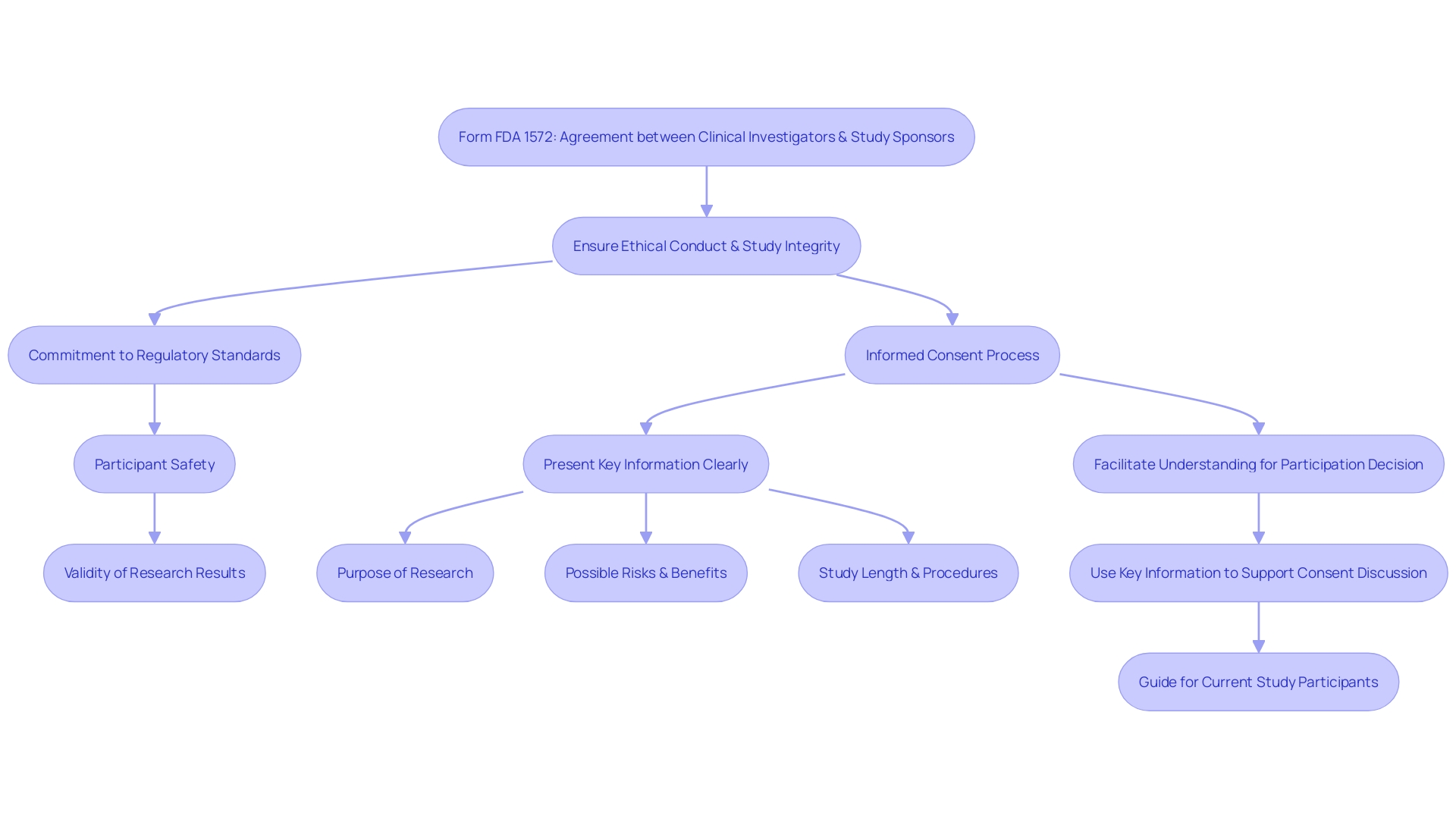
Form FDA 1572 is a critical document that establishes a formal agreement between clinical investigators and study sponsors in the realm of clinical research. The form signifies a commitment by the investigator to adhere to regulatory standards and guidelines, ensuring ethical conduct and integrity throughout the course of a study. It delineates the specific responsibilities an investigator must uphold, particularly in studies involving investigational new drugs (IND) and certain medical devices.
The importance of Form FDA 1572 is underscored by the FDA's role in safeguarding public health by ensuring the safety, effectiveness, and security of medical interventions. This includes rigorous oversight of human and veterinary drugs, biological products, medical devices, and more. The form serves as a cornerstone in maintaining the high standards expected in clinical trials, which is essential for the protection of study participants and the validity of research results.
As part of the informed consent process, which is integral to participant safety and ethical study conduct, the FDA emphasizes the clarity and conciseness of information provided to potential participants. The draft guidance on informed consent recommends presenting key study details upfront, such as research objectives, potential risks and benefits, and the overall structure of the study, to aid understanding and informed decision-making.
Moreover, the FDA's recent focus on innovation, such as the adoption of artificial intelligence (AI) and machine learning (ML) in clinical research, comes with an increased scrutiny of safety and compliance. Navigating the evolving regulatory landscape requires a careful balance between embracing cutting-edge technologies and ensuring participant welfare, as per the guidelines proposed by prominent regulatory bodies.
In light of these developments, the FDA continues to uphold the principles of the Declaration of Helsinki, a foundational ethical document for medical research that emphasizes the need for continuous ethical oversight. As the regulatory environment adapts to modern advancements, the Declaration's enduring principles guide the ethical conduct of research involving human participants.
It is essential for clinical investigators and all stakeholders involved in the clinical trial process to have a comprehensive understanding of the responsibilities and regulatory requirements encapsulated by Form FDA 1572. This ensures the collective aim of advancing medical knowledge while prioritizing patient safety and treatment outcomes.
What Information Must Be Provided on Form FDA 1572
The FDA Form 1572 is a cornerstone document in clinical research that delineates the responsibilities of principal investigators (PIs). It is crucial for PIs to provide comprehensive details including their qualifications and experience, which underscores their capability to conduct the trial. This form not only documents the Pi's commitment to adhere to FDA regulations but also lists sub-investigators and research coordinators, establishing a clear chain of accountability within the research team. In addition, it encapsulates essential aspects of the study such as protocol title, number, and sponsor information, ensuring transparency and traceability. The act of signing the form is not merely administrative; it represents the Pi's pledge to uphold participant rights, data integrity, and regulatory standards, which are fundamental to advancing medical science and safeguarding public health.
As participants submit comments on the 1572 form, the FDA emphasizes the importance of excluding confidential information from public postings. The agency's commitment to protecting individual privacy and proprietary data is paramount when considering submissions. Furthermore, the FDA's ongoing efforts to harmonize human subject protection regulations with the HHS Common Rule demonstrate a dedication to enhancing clinical research efficiency while maintaining rigorous participant safeguards. These initiatives contribute to the generation of reliable data that informs the FDA's decisions regarding medical product safety and efficacy, ultimately facilitating medical advancements.
In light of recent news, the FDA continues to assure the public of its mission to ensure the safety, effectiveness, and security of medical products. The agency's role extends to overseeing the nation's food supply, cosmetics, and other consumer products, reflecting its comprehensive responsibility for public health. The FDA's actions, from regulatory oversight to guidance on informed consent, are designed to foster an environment where medical progress thrives alongside robust participant protection.
Who Needs to Complete Form FDA 1572
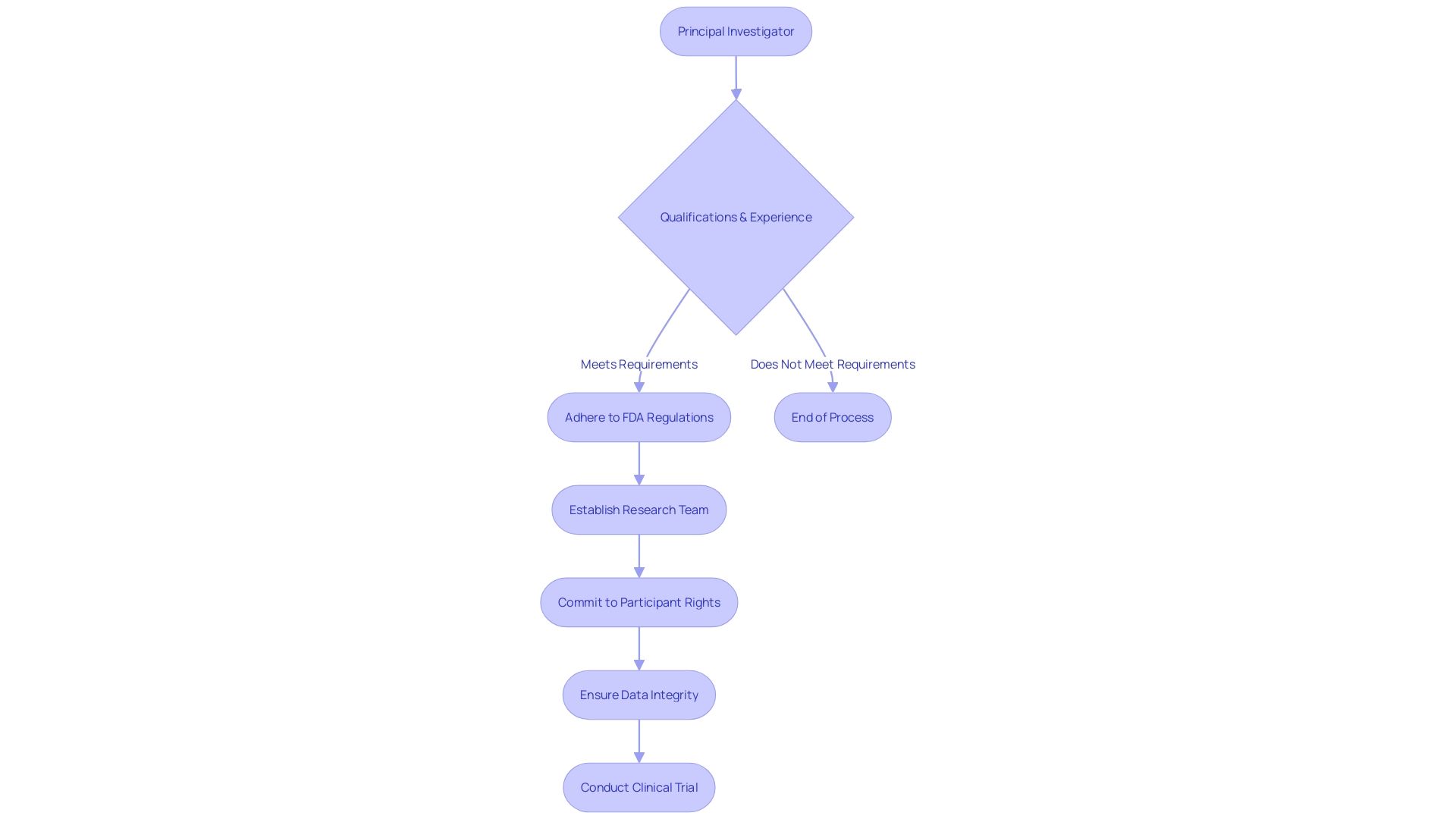
Principal investigators and lead researchers carry the crucial responsibility of completing the Form FDA 1572, a key document in clinical research that underscores their commitment to adhere to regulatory standards and oversee trial conduct. It is imperative for these professionals to possess the requisite qualifications and experience to execute clinical trials effectively. Occasionally, the role extends to sub-investigators and clinical research coordinators who may be charged with filling out specific sections of the form. Acknowledging the gravity of this task, it is paramount that all individuals involved in completing the Form FDA 1572 are well-versed with the regulatory necessities and their duties as investigators to ensure the integrity and safety of clinical research.
The FDA emphasizes the importance of safeguarding confidential information when submitting comments or documents related to the form, reflecting the agency's commitment to maintaining the security of sensitive data in compliance with legal and ethical standards. Chris, a biomedical engineer with extensive experience managing clinical trials for Class III medical devices, exemplifies the caliber of expertise necessary for those involved in the submission and management of Form FDA 1572. His work as a Solutions Engineer further highlights the interdisciplinary nature of clinical research and the need for adept professionals to navigate its complexities.
The FDA's role in assuring the safety and efficacy of medical products extends to the review of submissions like the Form FDA 1572, ensuring that clinical trials are conducted in accordance with the highest standards. As the agency responsible for the oversight of drugs, medical devices, and other health-related products, the FDA's regulations are integral to the protection of public health, underscoring the significance of meticulous completion and management of the form by qualified investigators.
When Must Form FDA 1572 Be Completed and Signed
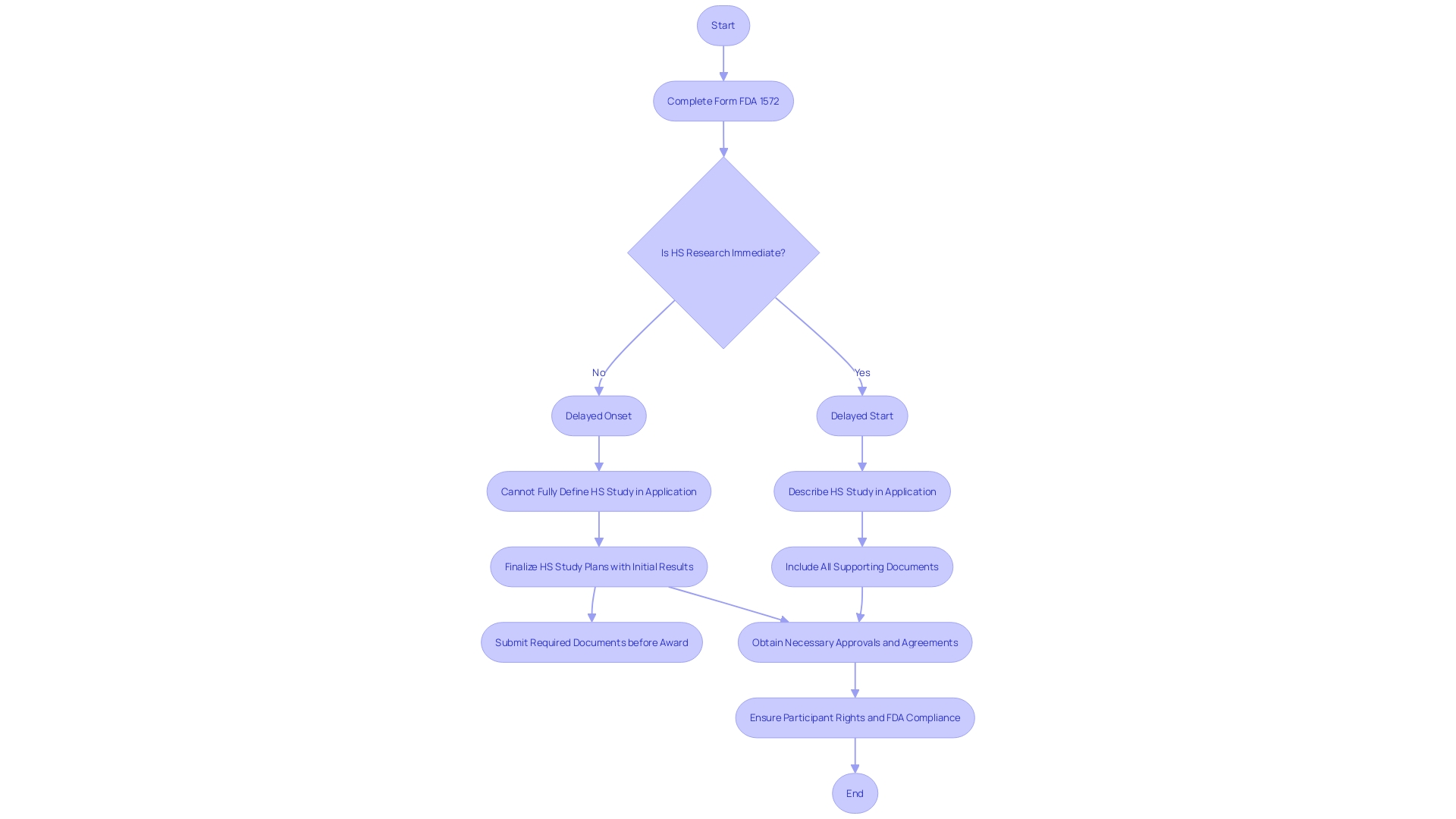
Before commencing any clinical research study, it is imperative for investigators to complete and sign Form FDA 1572. This form is not only a formal attestation to the investigator's readiness to adhere to regulatory standards and guidelines but also a critical document that underlines their commitment to uphold the integrity of the research process. The signing of Form FDA 1572 is a key step during the study startup phase, signifying that the investigator has secured all necessary approvals and agreements, aligning with the principles of good clinical practice. It is crucial to distinguish between 'delayed start' and 'delayed onset' studies, as the former can be fully described in the research application, while the latter requires initial research results to define the human subjects study at a later stage. Misclassifying these terms in applications could lead to significant omissions, affecting the study's assessment or potentially resulting in a bar to award. This form is a linchpin in the safeguarding of participant rights and well-being, and its completion is a testament to the FDA's mission to ensure the safety, efficacy, and security of medical interventions.
Submission and Updating of Form FDA 1572
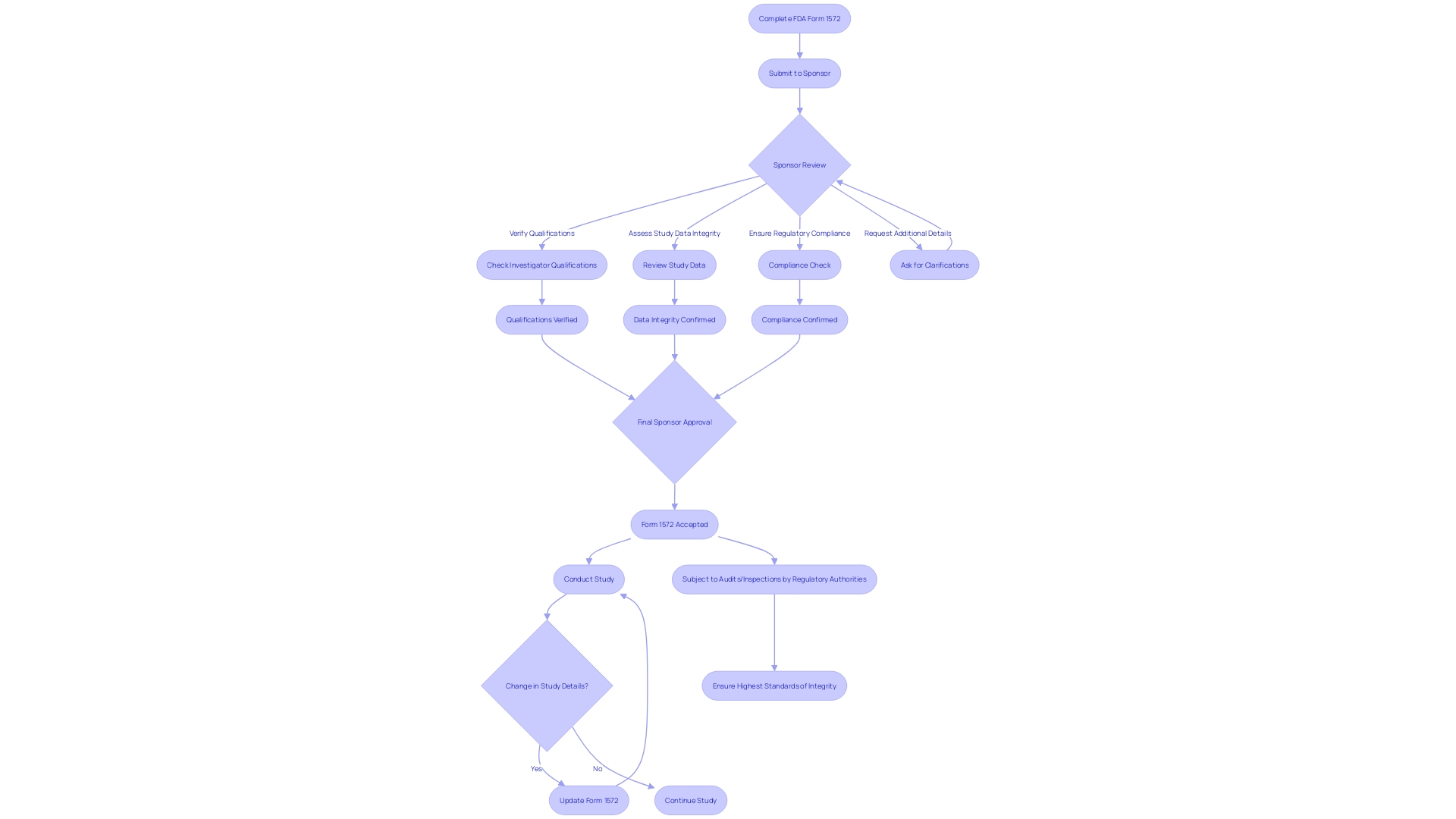
Upon completion, the FDA Form 1572 is submitted to the clinical study's sponsor for review. This critical document ensures that the sponsor can verify the investigator's qualifications, the integrity of study data, and compliance with regulatory requirements. The sponsor may also request supplementary details or clarifications, if necessary. Regulatory authorities, too, have the prerogative to examine this form during audits or inspections, reinforcing its importance in the research process. It is the responsibility of the investigator to maintain the currency of Form 1572—updating it promptly should there be changes in study personnel, investigator qualifications, or contact information. This meticulous attention to detail ensures the study upholds the highest standards of integrity, reflecting the FDA's commitment to public health and safety.
Exemptions and Special Cases
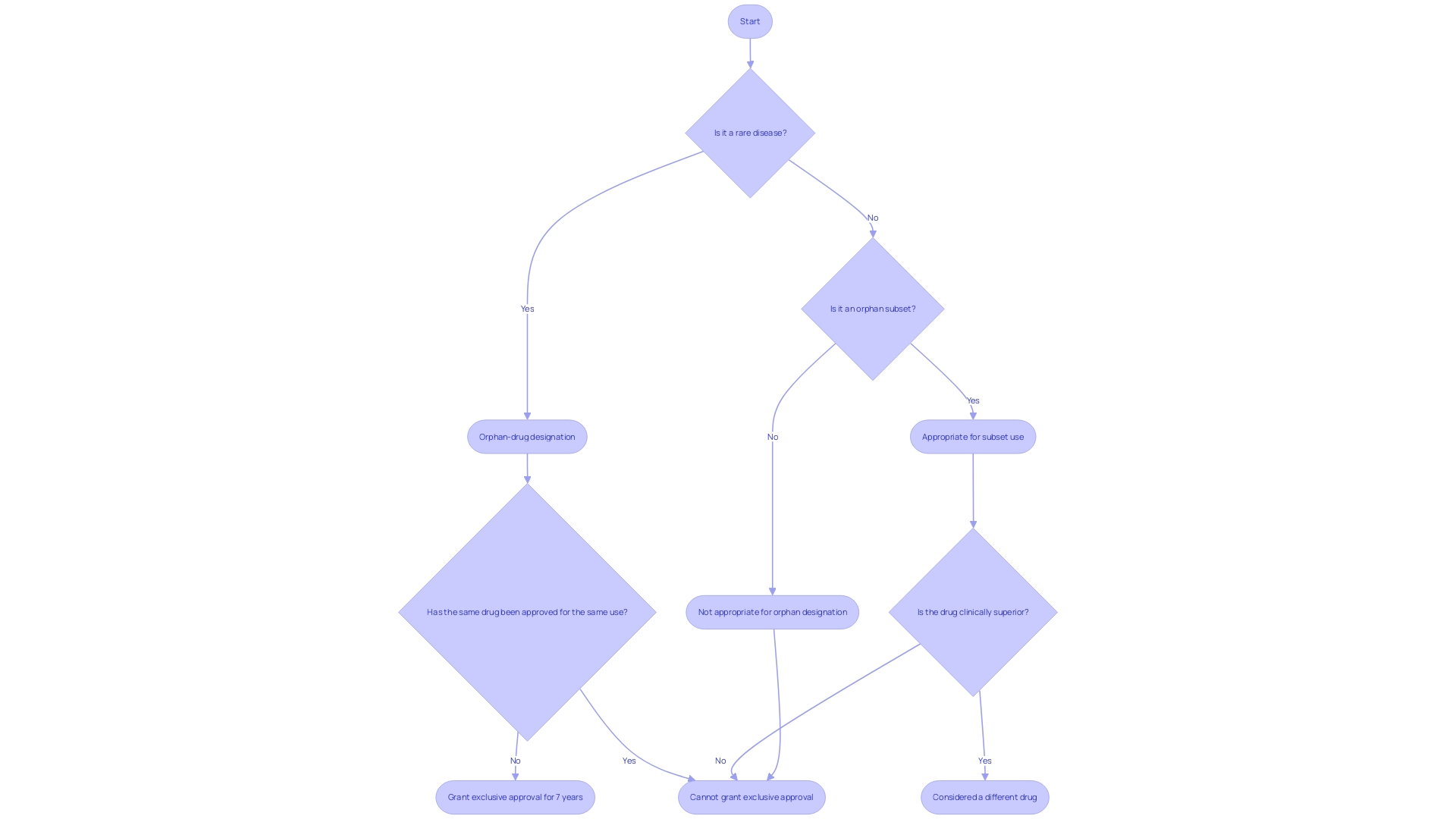
Navigating the complexities of clinical research regulations requires a thorough understanding of when specific documentation, such as Form FDA 1572, is needed. Although this form is a staple for most Investigational New Drug (IND) studies and select device trials, there are notable exceptions and scenarios where it may not be mandatory. For instance, orphan drugs—those aimed at treating rare diseases or conditions—may operate under different rules, as stipulated by section 526 of the Federal Food, Drug, and Cosmetic Act. These drugs, upon receiving orphan-drug designation, are granted exclusive approval for seven years post-FDA approval, during which similar drugs cannot be approved for the same indication unless clinically superior.
Furthermore, a drug intended for an 'orphan subset' of a non-rare disease might be exempt due to unique characteristics, such as its toxicity or mechanism of action. It's essential for investigators to familiarize themselves with these exemptions to ensure compliance and to determine the applicability of Form FDA 1572 for their study. They should consult current regulations or seek advice from the study's sponsor or regulatory authorities, as the landscape of clinical research is continually shaped by ethical, legal, and social considerations, as well as market incentives and intellectual property rights.
Recent studies highlight the gravity of adherence to such protocols, showing that a significant portion of clinical trials fail to report results or maintain a public record, as evidenced by a study reviewing over 6,700 trials in Canada. This underscores the need for meticulous record-keeping and transparency in clinical research, a responsibility that Form FDA 1572 helps to enforce when applicable.
Common Mistakes and Best Practices in Filling Out Form FDA 1572
Accurate completion of Form FDA 1572 is not just a bureaucratic step; it's an essential part of maintaining regulatory compliance in clinical research. This form, often referred to as the 'Statement of Investigator', is a binding document that confirms the investigator's commitment to follow the FDA's regulations and to conduct the study in accordance with the study protocol. Errors such as incomplete information, missing signatures, or outdated forms can result in significant setbacks, not just in terms of administrative efficiency but also in ensuring the safety and efficacy of the research being conducted.
To ensure the integrity of the clinical trial process, it's imperative to provide all required information meticulously. This can be a complex task, as exemplified by a case where a firm neglected to perform identity tests for each component of a drug product, specifically those at high risk of contamination such as glycerin and propylene glycol. This oversight, a violation of 21 CFR 211.84(d)(1), highlights the critical nature of thorough testing and documentation.
Moreover, it's important to note that the FDA continuously strives to safeguard public health by enforcing rigorous safety measures. Consequently, the role of Form FDA 1572 extends beyond mere compliance; it underpins the very trust in the safety, effectiveness, and security of medical interventions.
Given these challenges, investigators are encouraged to seek guidance from seasoned professionals who are well-versed in regulatory requirements. Keeping abreast of updates to regulations is equally important, as the landscape of clinical research is ever-evolving. Training and clear, organized documentation are also best practices that can help avoid common pitfalls.
For instance, the FDA's draft guidance on informed consent emphasizes the importance of clear communication, suggesting that key information be presented at the outset of consent documents. This approach can serve as a valuable reference for investigators when compiling necessary details for Form FDA 1572.
Ultimately, the responsibility lies with the investigators to maintain the highest standards of accuracy and compliance. This includes a proactive approach to managing safety concerns in drug development, as outlined in training courses aimed at healthcare professionals and individuals involved in biomedical research. By adhering to these practices, investigators can contribute to the advancement of medical science while upholding the ethical and legal standards that protect research participants and the integrity of clinical data.
Conclusion
Form FDA 1572 is a critical document in clinical research that establishes a formal agreement between investigators and sponsors. It ensures adherence to regulatory standards, ethical conduct, and integrity throughout the study. Principal investigators and lead researchers are responsible for completing the form, with accurate completion and timely updates being crucial for regulatory compliance.
Before commencing any clinical research study, the form must be completed and signed, signifying the investigator's commitment to regulatory standards and participant rights. It is then submitted to the study's sponsor for review, allowing verification of qualifications and compliance with regulations. Understanding exemptions and special cases where the form may not be mandatory is important for compliance and determining its applicability.
To ensure accuracy, it is best practice to provide all required information meticulously, seek guidance from experienced professionals, and stay updated on regulations. Training, clear documentation, and adherence to ethical and legal standards are vital for participant safety and the integrity of clinical data.
In conclusion, Form FDA 1572 plays a crucial role in clinical research by ensuring adherence to regulatory standards and ethical conduct. Accurate completion and timely updates are essential for maintaining regulatory compliance and safeguarding participant safety. By upholding the highest standards of accuracy and compliance, investigators contribute to the integrity and trust in the safety, effectiveness, and security of medical interventions.




