Introduction
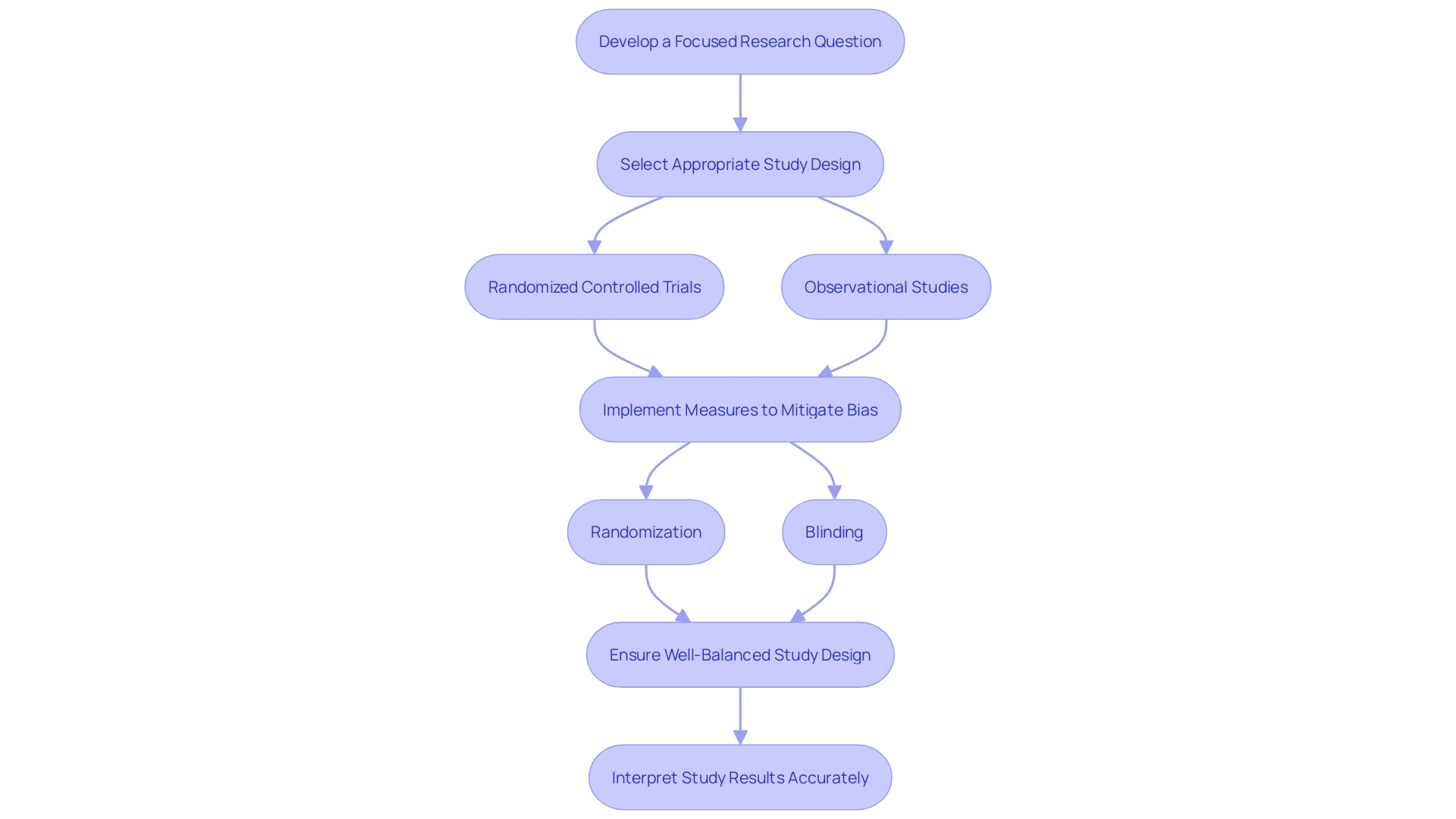
Rigorous study designs are essential for the integrity of pivotal studies that aim to determine the efficacy of new interventions. The randomized controlled trial (RCT), considered the gold standard, utilizes randomization and blinding to mitigate bias and ensure accurate results. A well-balanced study design is crucial, as highlighted by cases where imbalances in control and active arms raised questions about statistical adjustments.
Developing a focused research question centered on the population, intervention, comparison, and outcome is also critical. Ethical considerations play a vital role in pivotal studies, with guidelines like the Declaration of Helsinki emphasizing respect for individuals and justice. Innovative approaches to informed consent, such as the use of videos and illustrations, accommodate diverse participant needs.
Pivotal studies have a significant impact on clinical practice, shaping future medical interventions and enhancing patient outcomes. They can lead to the inclusion of new drugs in standard treatment protocols and identify unforeseen health issues. However, conducting pivotal studies comes with challenges, including participant recruitment and maintaining engagement.
Aligning research with clinical practice and ensuring diversity in participant representation are ongoing priorities. Overall, pivotal studies are instrumental in advancing medical knowledge and improving patient care.
The Importance of Rigorous Study Design
Rigorous study designs are fundamental to the integrity of pivotal studies, which aim to determine the efficacy of new interventions. One such design is the randomized controlled trial (RCT), a gold standard that employs randomization and blinding to mitigate bias and confounding variables, ensuring that the results reflect the true effects of the intervention. A study's power is also crucial, as highlighted by a case where a control arm was disproportionately smaller than the active arm, raising questions about the validity of the statistical adjustments made. This underscores the importance of a well-balanced study design, which is as critical as the research question itself. Developing an answerable and focused research question is essential, centering on the specifics of the population, intervention, comparison, and outcome. Observational studies, another study design, use the term 'exposure' to describe a factor's association with an outcome of interest, such as the exposure of smoking to the outcome of lung cancer. The clarity of the research question directly influences the methodology deployed. Moreover, the interpretation of study results can be challenging, as complex data displays in healthcare provider-targeted prescription drug advertisements have shown to be often misunderstood. These intricate aspects of study design and result interpretation contribute to a comprehensive understanding of pivotal clinical trials and their findings.
Ethical Considerations in Pivotal Studies
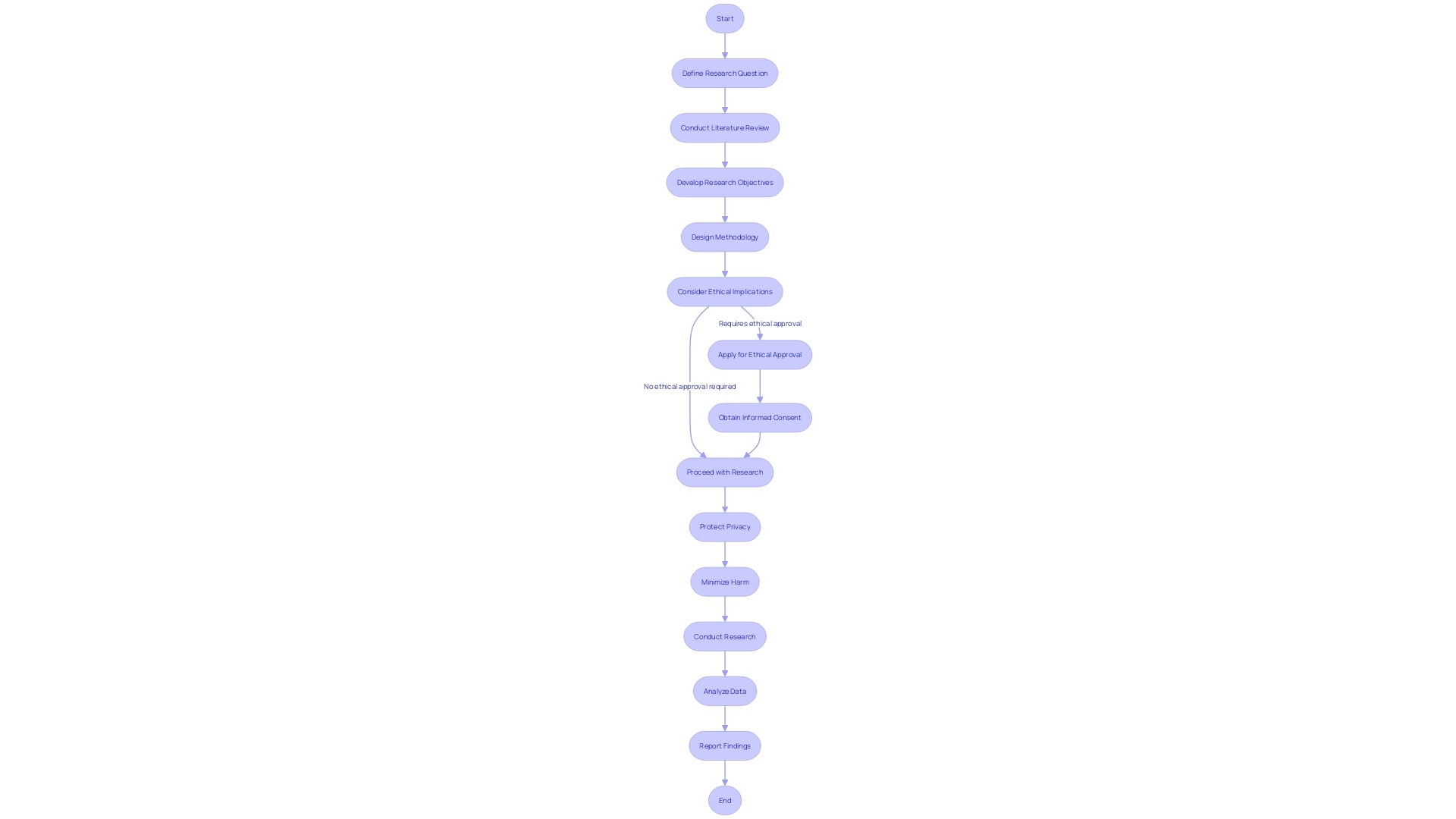
Maintaining high ethical standards is critical in pivotal studies to ensure the trustworthiness of the findings. A taxonomy has been established to clarify key terms in research ethics, aiding in the understanding of concepts such as informed consent, privacy protection, and harm minimization. For example, informed consent involves not only the initial agreement to participate but also ongoing consent throughout the research process. The Declaration of Helsinki and the Belmont Report provide foundational ethical guidelines emphasizing respect for individuals, beneficence, and justice.
To assist researchers in upholding these principles, self-assessment tools have been developed to determine when ethical approval is required, especially when engaging with the public. A code of conduct also offers a framework for respectful research practices. Ethical review boards play an instrumental role in this process, safeguarding the rights and welfare of participants.
Innovative approaches to informed consent, such as the use of videos and illustrations, have been endorsed by organizations like the National Organization for Rare Disorders and the Pharmaceutical Research and Manufacturers of America. These methods accommodate diverse participant needs, ensuring comprehension across varying levels of health literacy and physical abilities.
Despite advancements in ethical frameworks and tools, continuous efforts are necessary to enhance fairness and respect in research. Evolving practices, including the potential role of artificial intelligence in ethical decision-making, indicate a dynamic landscape where ethical vigilance remains paramount.
The Impact of Pivotal Studies on Clinical Practice
Pivotal studies serve as cornerstones in clinical research, shaping the future of medical practice. These rigorously conducted trials provide robust evidence on the safety and effectiveness of new medical interventions. Through pivotal studies, healthcare professionals receive critical data that underpin evidence-based decisions critical for patient care. For instance, the B-HAT and ISIS 1 trials on beta-blockers brought about a paradigm shift, showing a 3% absolute risk reduction in mortality, which was highly significant statistically. This kind of evidence can lead to the inclusion of new drugs in standard treatment protocols, ultimately enhancing patient outcomes and care quality.
Clinical trials also play a vital role in identifying unforeseen health issues, as evidenced by participants like Barbara, who discovered her heart condition through involvement in a study found via The New Normal platform. Such platforms have revolutionized participant recruitment, offering access to trials that not only advance medical knowledge but also provide individual health insights.
In the realm of treatment for chronic conditions, large-scale pivotal trials can confirm or refute the efficacy of off-label medication use, such as in the case of mirtazapine for breathlessness in respiratory diseases. These findings, while sometimes disappointing, are indispensable in refining patient care strategies and preventing unnecessary side effects.
Moreover, the evolution of healthcare delivery necessitates that clinical trial results are disseminated effectively, aligning with the 'Five Rights' framework of Clinical Decision Support (CDS) – ensuring the right information reaches the right individual at the right time and in the right manner. This is crucial as care settings diversify and the healthcare workforce sees a shift towards more advanced practice clinicians being the first point of contact for patients.
Statistics in Medicine's 40th-anniversary celebrations underscore the ongoing importance of biostatistics in interpreting clinical trial data. As the medical community continues to scrutinize research quality and reporting, the significance of pivotal studies in informing clinical practice and improving patient outcomes remains as relevant as ever.
Case Study: Successful Implementation of a Pivotal Study
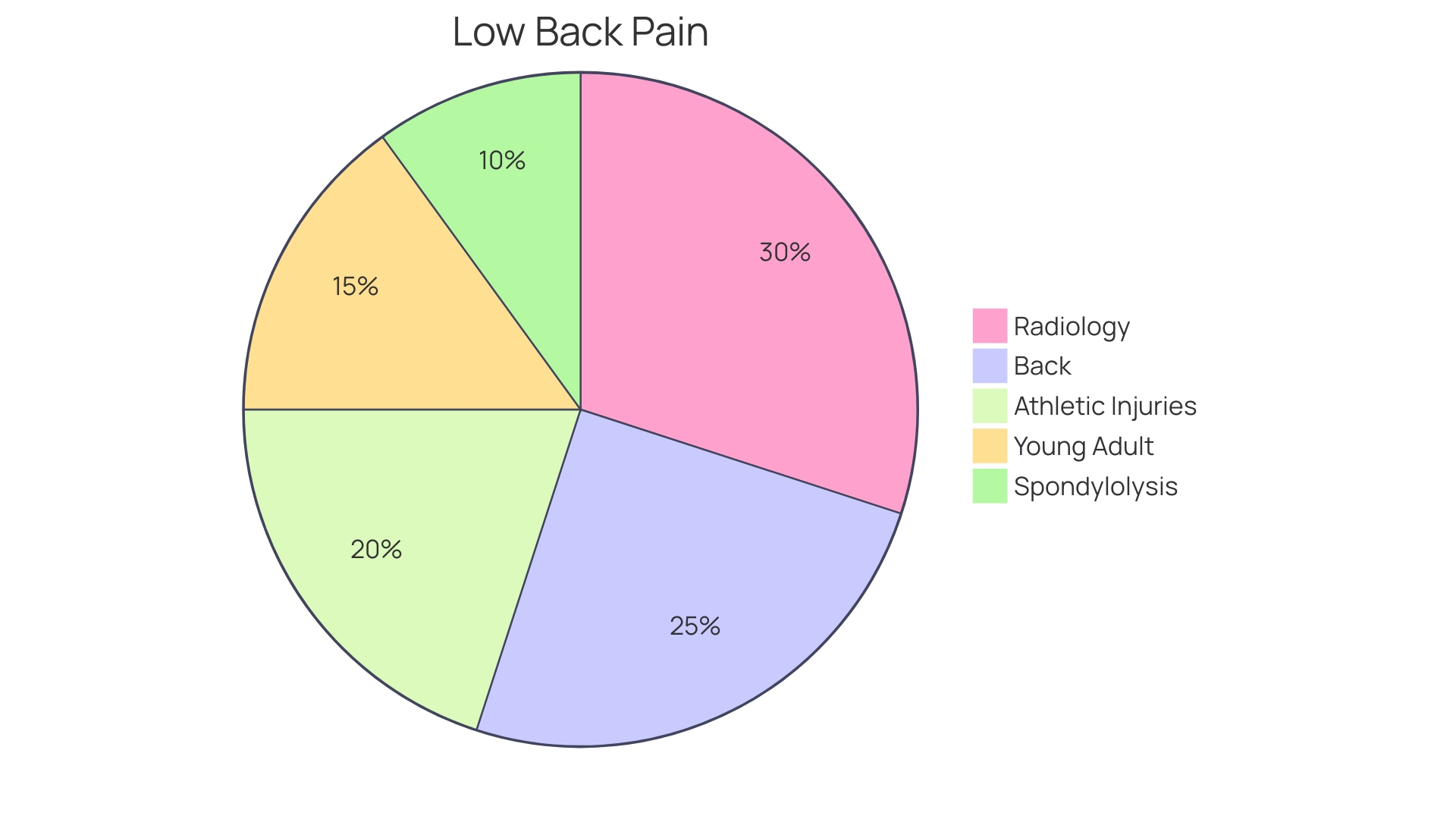
A recent study published in the Journal of the American College of Cardiology has brought to light a groundbreaking surgical technique aimed at addressing spondylolisthesis, a condition contributing significantly to the global burden of lower back pain. Spondylolisthesis, characterized by the slippage of one vertebra over another, particularly in the lower spine, not only leads to pain which can intensify during activities such as walking or standing but also may cause sciatica, with symptoms including numbness and tingling sensations radiating down the leg.
The study's importance is underscored by the startling statistics reported by the World Health Organization, which noted that in 2020, lower back pain affected 619 million individuals worldwide, making it the top cause of disability across the globe.
The multicenter study involved a comprehensive sample size, providing a robust assessment of the novel surgical intervention's efficacy. The results were promising, showcasing a significant enhancement in patient outcomes post-surgery. This success story illustrates not only the potential for improved quality of life for those suffering from spinal conditions but also emphasizes the value of rigorous clinical trials in the advancement of medical practices. The ripple effect of this pivotal study's findings has been the wider adoption of the surgical technique in clinical settings, offering hope and improved health prospects to countless patients.
Challenges and Limitations in Conducting Pivotal Studies
Pivotal studies serve as cornerstones in medical research, yet they come with a unique set of challenges that researchers must navigate. Recruitment of participants is a primary hurdle, often limited by time constraints which can impede the progress of a study. The commitment to maintain participant engagement throughout the study is also a demanding task, as researchers work to minimize dropout rates and enhance compliance. Financial and logistical hurdles further complicate the execution of large-scale pivotal studies. However, the pursuit of innovative solutions is relentless among researchers, who aim to strengthen the robustness of their studies despite such obstacles.
As noted by Derek Angus and colleagues in a JAMA publication, the gap between clinical trial design and the realities of clinical practice often results in inefficiencies and limited impact of trials. Such discrepancies highlight the importance of aligning research with practice to improve health outcomes. The challenge is underscored by the substantial number of registered RCTs, which, as reported, amount to 40,000 annually on ClinicalTrials.gov, yet still leave considerable clinical uncertainty. This reinforces the necessity to refine trial designs and ensure that results are applicable and beneficial to the wider population.
Furthermore, the inclusion of diverse populations in clinical trials is paramount. Studies have indicated that trials predominantly based in the USA fail to represent the true ethnic diversity of the affected populations, compromising the generalizability of results. This lack of representation can perpetuate health disparities and undermine efforts towards health equity, as stated in reviews analyzing the recruitment and retention of ethnic diversity in trials. Therefore, researchers are tasked with the essential responsibility of recruiting diverse and representative participant groups, acknowledging the complexity of ethnicity as a multi-faceted and context-specific construct, to validate the findings and uphold the principles of health justice.
Conclusion
Rigorous study designs, like randomized controlled trials (RCTs), are crucial for determining the efficacy of new interventions. Ethical considerations, including innovative approaches to informed consent, ensure the trustworthiness of pivotal study findings. Pivotal studies have a significant impact on clinical practice, shaping future interventions and enhancing patient outcomes.
They can lead to the inclusion of new drugs in treatment protocols and identify unforeseen health issues. However, conducting pivotal studies comes with challenges, such as participant recruitment and engagement. Researchers must navigate financial and logistical obstacles to strengthen the robustness of their studies.
In conclusion, pivotal studies advance medical knowledge and improve patient care. Rigorous study designs and ethical considerations are key priorities. Despite challenges, the commitment to high-quality studies ensures valid findings, benefiting the wider population and upholding health justice principles.
Overcome the challenges of conducting pivotal studies with our expert guidance and support.




