Overview
Accelerating research in Latin America requires a multifaceted approach that includes investing in infrastructure, fostering collaborations between academia and industry, and navigating regulatory frameworks effectively. The article emphasizes that leveraging the region's unique advantages, such as its diverse populations for clinical studies and the increasing role of the business sector in funding, is essential for overcoming challenges like limited funding and bureaucratic hurdles, ultimately positioning Latin America as a leader in global research initiatives.
Introduction
In the vibrant tapestry of Latin America's research landscape, a unique blend of challenges and opportunities shapes the future of scientific inquiry. With institutions like INVIMA leading the charge in regulatory excellence, the region stands at a crossroads, where innovation is both a beacon of hope and a daunting hurdle.
As researchers navigate the complexities of funding, regulatory frameworks, and workforce development, they must also harness the rich diversity of populations and emerging technologies to drive impactful clinical studies.
This article delves into the current state of research in Latin America, exploring strategic priorities for enhancing capabilities, navigating regulatory environments, and building effective partnerships that can propel the region into a new era of scientific advancement.
The Current Landscape of Research in Latin America
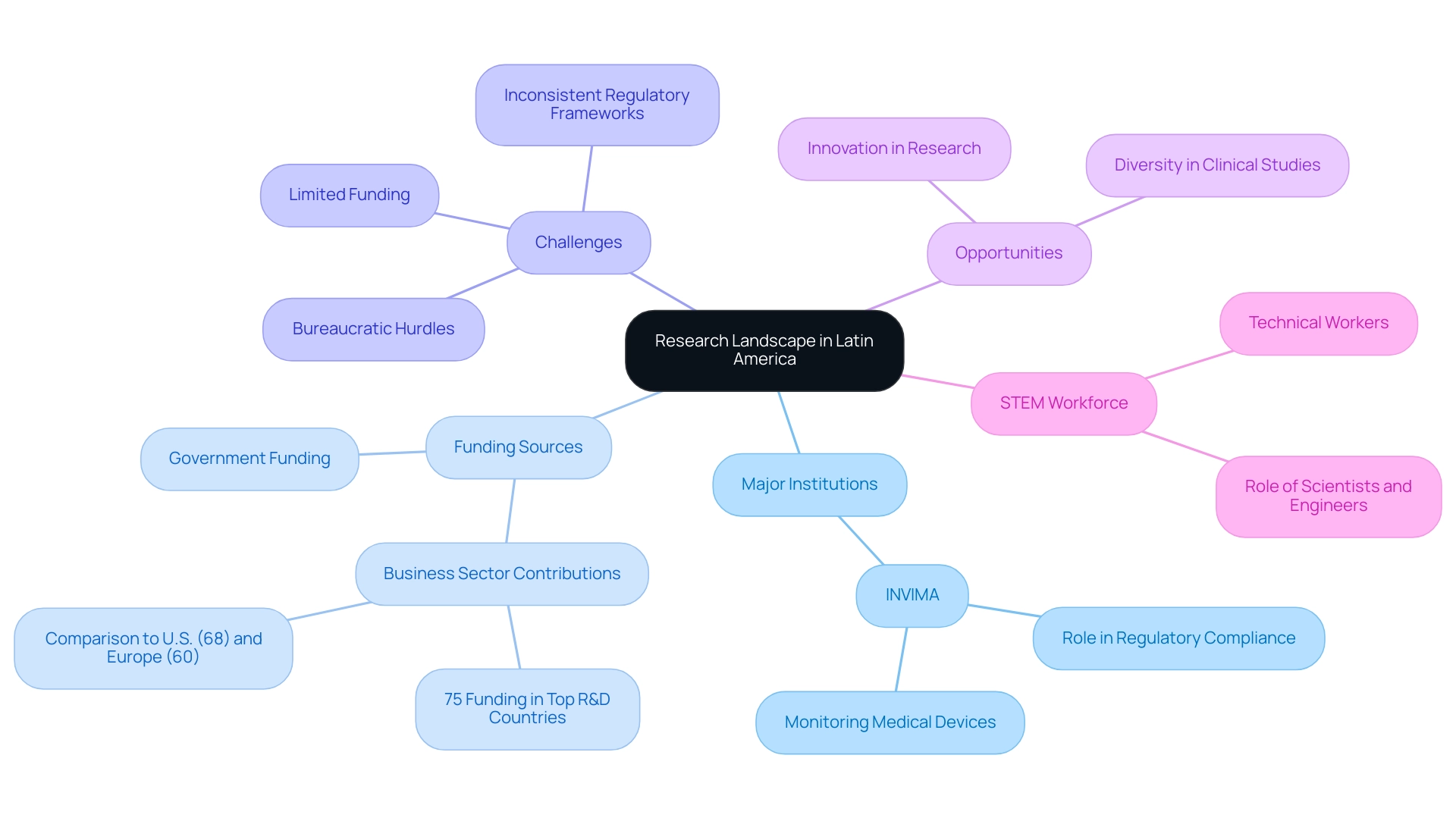
Latin America showcases a vibrant and multifaceted academic landscape that is pivotal in accelerating research in Latin America, blending both advanced and emerging institutions dedicated to scientific inquiry. A critical player in this ecosystem is INVIMA (Colombia National Food and Drug Surveillance Institute), established in 1992, which oversees the marketing and manufacturing of health products, ensuring compliance with safety and efficacy standards. The Directorate for Medical Devices and other Technologies within INVIMA plays a vital role in monitoring and controlling medical devices, suggesting technical standards for their manufacturing and marketing, and ensuring quality assurance.
As a Level 4 health authority acknowledged by PAHO/WHO, INVIMA's regulatory excellence is essential for promoting clinical trials and innovation in the area. However, the region grapples with significant challenges that can impede progress in accelerating research in Latin America, notably limited funding, bureaucratic hurdles, and inconsistent regulatory frameworks. Recent observations suggest that interest payments in LAC and the Caribbean have surpassed essential government expenditures over the past decade, complicating the financial environment for scholarly initiatives.
In contrast, the business sector emerges as a crucial funding source, as evidenced by case studies showing that in top R&D-performing countries, domestic businesses contribute at least 75% of funding, a stark contrast to the U.S. at 68% and European countries at around 60%. This disparity highlights the significance of enhancing the business sector's role in financing studies in South America. Navigating these complexities is essential for researchers focused on accelerating research in Latin America, as they must also leverage the region's unique advantages, such as its rich diversity of populations, which is invaluable for clinical studies.
Moreover, a growing interest in innovation signals potential for accelerating research in Latin America. As noted by the OECD, 'The business sector is the largest funder of R&D in the top R&D-performing countries, with lower shares funded by government, higher education, and private nonprofit institutions.' Furthermore, in 2021, the National Institutes of Health (NIH) backed approximately 23,000 S&E graduate students, while the NSF assisted nearly 22,000 students, emphasizing the extent of support for educational endeavors that contrasts with the funding challenges encountered in America.
Grasping these dynamics, including the essential role of scientists, engineers, and proficient technical workers from the STEM workforce, is vital for accelerating research in Latin America and developing effective strategies to expedite initiatives throughout Central and South America. Moreover, media coverage has progressively emphasized the significance of clinical trials in the region, illuminating INVIMA's role in ensuring regulatory compliance and promoting a favorable atmosphere for clinical studies.
Strategies for Enhancing Research Capabilities
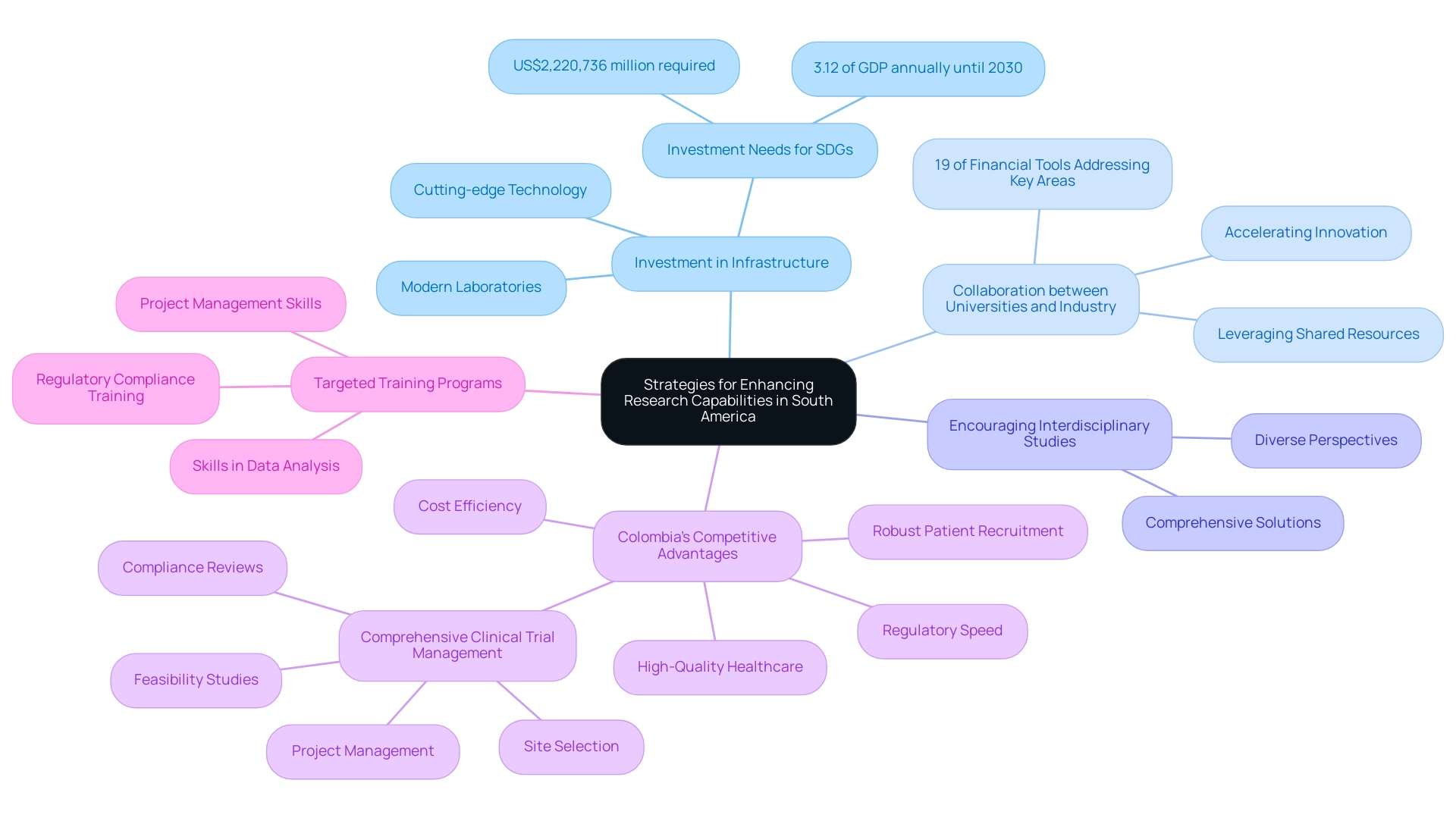
To greatly improve investigative abilities in South America, institutions must prioritize accelerating research in Latin America along with several strategic priorities.
- Investing in modern development infrastructure—such as advanced laboratories and cutting-edge technology—is crucial for fostering innovation. This corresponds with the results from the 'Investment Needs for Sustainable Development Goals in Latin America and the Caribbean,' which estimates that the region needs to invest approximately US$2,220,736 million across various sectors, including studies, to meet the SDGs by 2030.
- Fostering collaborations between universities and industry can create synergies that leverage shared resources and expertise, thereby accelerating the pace of innovation. Significantly, merely 19% of financial tools suggested by development finance organizations tackle essential areas such as digital transformation, highlighting the necessity for more cooperative structures to boost funding in innovation.
- Interdisciplinary studies must be encouraged to effectively tackle complex health issues, as this approach brings together diverse perspectives and expertise to create comprehensive solutions.
Additionally, Colombia’s competitive advantages—including cost efficiency, regulatory speed, high-quality healthcare, robust patient recruitment capabilities, and comprehensive clinical trial management services such as feasibility studies, site selection, compliance reviews, and project management—position it as a favorable destination for first-in-human clinical trials.
Lastly, implementing targeted training programs is essential for equipping researchers with vital skills in data analysis, project management, and regulatory compliance. As William Maloney pointed out during a recent conversation, grasping investment trends and emerging economic prospects is essential for progressing the area's academic environment.
By concentrating on these strategies, institutions in South America can foster a strong investigative ecosystem that plays a crucial role in accelerating research in Latin America, tackling urgent health issues and positioning the region as a frontrunner in global initiatives.
Navigating Regulatory Frameworks for Efficient Research
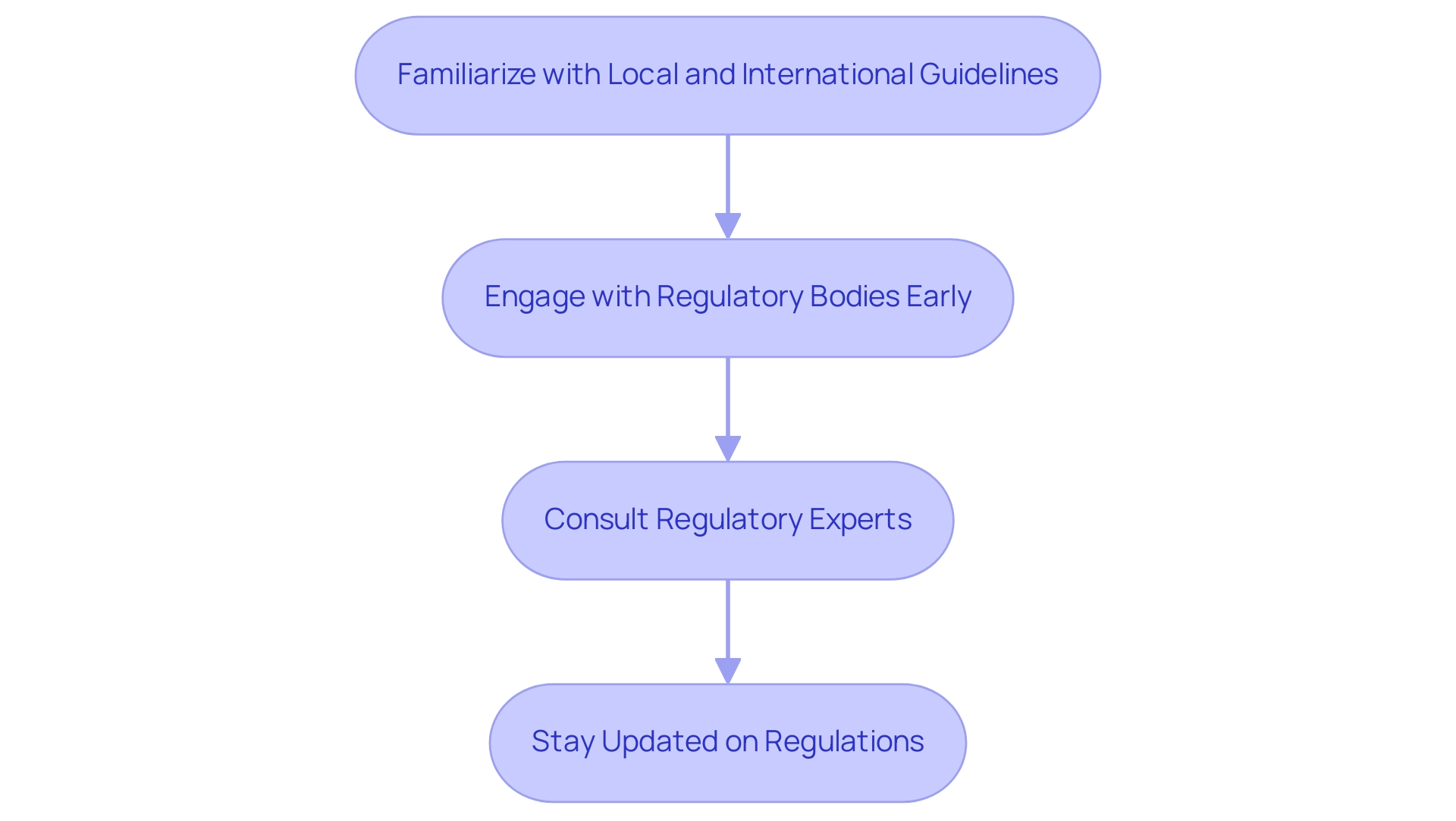
Skillfully maneuvering through regulatory structures is essential for investigators focused on accelerating research in Latin America to expedite medical studies and innovation. To do so, researchers should adopt several best practices:
- Become well-acquainted with both local and international guidelines pertinent to their studies, ensuring compliance from the outset.
- Engage proactively with regulatory bodies early in the process to clarify requirements and expectations.
- Leverage the expertise of regulatory consultants or legal advisors who specialize in clinical studies to navigate complexities.
- Remain vigilant about updates in regulations that could impact protocols.
This proactive strategy not only streamlines the approval process but also enhances the overall efficiency of research activities.
Significantly, ten countries in the LAC area have established at least one digital platform aimed at enhancing participation in public consultations, illustrating effective engagement with regulatory processes. Given the current inflationary pressures in the area, which have heightened concerns about price stability and could affect regulatory environments, staying informed is more vital than ever. As articulated by Juan Ignacio Lacapmesure, Economic Analysis Assistant at Deloitte LATAM Argentina, 'However, this recession scenario remains speculative at present according to the latest speech of the Chair of the Federal Reserve of the United States.'
This uncertainty in the economic landscape underscores the importance for researchers to remain adaptable and informed in their compliance efforts. Additionally, as Steve Garchow emphasized in a recent podcast, understanding the unique challenges and structural issues in each country is essential for effective market access. Collaborating with bioaccess®, a reliable Contract Research Organization, can aid in accelerating research in Latin America by streamlining medical device research trials through extensive local knowledge and support.
Furthermore, bioaccess® is committed to information security in its operations, implementing reasonable measures to protect sensitive data, while also acknowledging the inherent risks involved. Researchers are encouraged to stay engaged with bioaccess® for comprehensive support in navigating the complexities of clinical trials in the region.
Building Effective Partnerships for Research Advancement
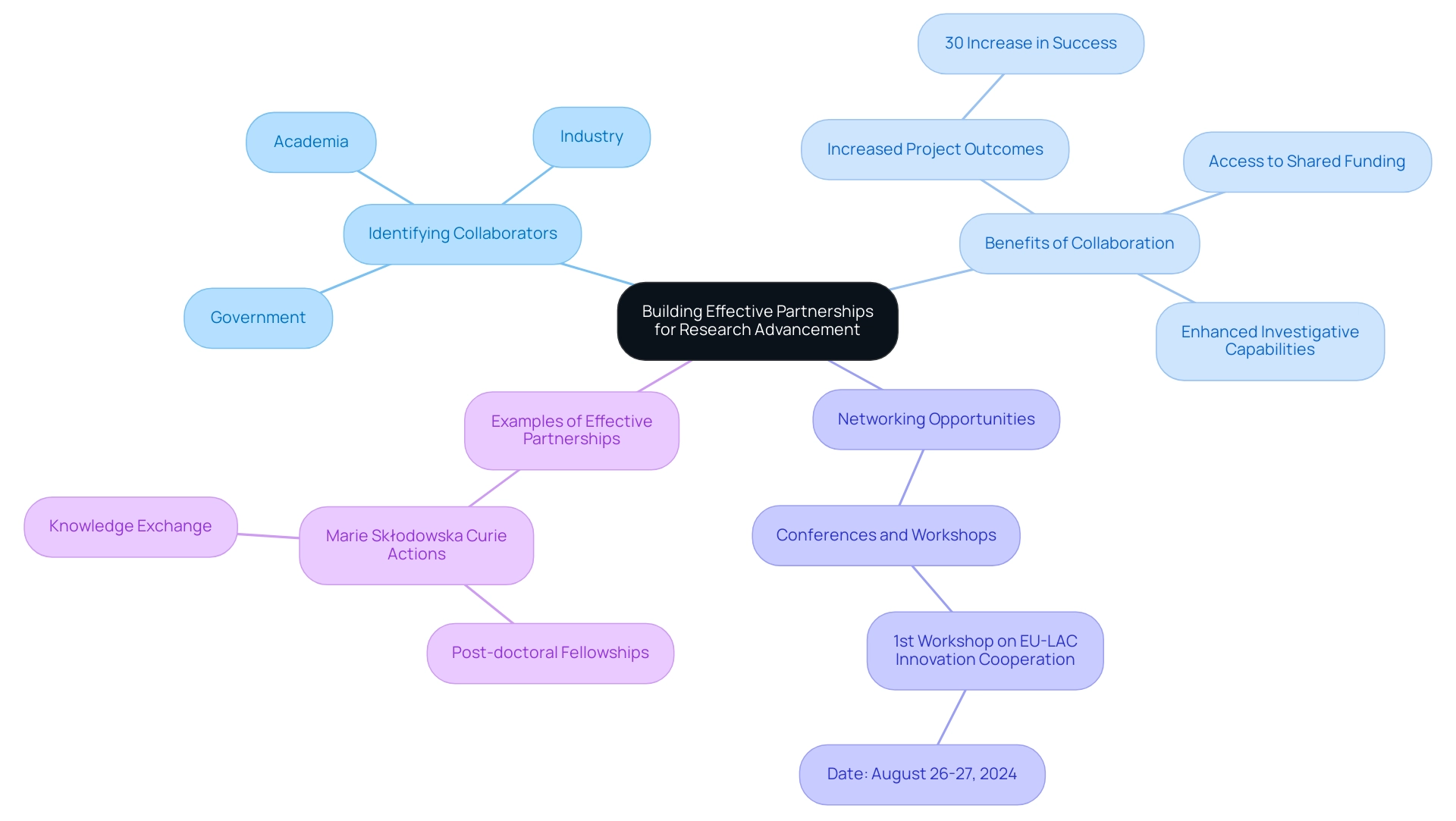
To cultivate effective collaborations in studies, it is essential for scholars to adopt a strategic approach. First, identifying potential collaborators across academia, industry, and government is crucial for leveraging diverse expertise. According to the Horizon Dashboard, partnerships in EU Research and Innovation Programs have shown a 30% increase in successful project outcomes when diverse stakeholders collaborate.
Actively participating in conferences and networking events, such as the recent 1st workshop on EU-LAC Innovation Cooperation held in Bogota on August 26-27, 2024, provides valuable opportunities to establish connections. A significant instance of effective international cooperation, which is crucial for accelerating research in Latin America, is the Marie Sklodowska Curie Actions, allowing post-doctoral scholars from Latin America and the Caribbean to spend up to two years in European academic teams, thereby boosting capacity and knowledge exchange. Moreover, Medtech clinical studies carried out in these areas generate waves with extensive economic advantages, such as job creation, healthcare enhancements, and heightened development, thereby fostering international acknowledgment.
Creating collaborative proposals that capitalize on the unique strengths of each partner can significantly enhance project outcomes. Moreover, maintaining open lines of communication and defining clear roles and responsibilities fosters a collaborative environment. These partnerships not only facilitate access to shared funding opportunities but also enhance investigative capabilities and outcomes, ultimately driving innovation and accelerating research in Latin America.
As Carolina Franco, Guest Editor, emphasizes, 'Effective partnerships are the cornerstone of impactful projects.' The strength of a partnership often lies in the synergy created through collaboration, allowing for more impactful initiatives that contribute to local economic growth and global health improvement, while also gaining international recognition.
Securing Funding to Propel Research Initiatives
Securing funding for investigative initiatives in Latin America is essential for accelerating research in Latin America, requiring a strategic and multifaceted approach, particularly in light of the significant regulatory framework established by INVIMA, the Colombia National Food and Drug Surveillance Institute. As a Level 4 health authority acknowledged by PAHO/WHO, INVIMA plays a crucial role in overseeing medical devices and ensuring compliance with health standards, thereby facilitating a supportive environment for clinical studies. INVIMA employs rigorous methodologies for evaluating medical devices, including pre-market assessments, post-market surveillance, and compliance checks to ensure that products meet established safety and efficacy standards.
Researchers should begin by identifying potential funding sources, which may include government grants, private foundations, and industry partnerships, all of which are critical in a region experiencing rapid growth in innovation.
A recent analysis by Dealroom highlights the emergence of the top 100 startups in LATAM, indicating a vibrant ecosystem that is essential for accelerating research in Latin America and collaboration. This surge in startups not only signifies a growing interest in innovation but also presents new opportunities for researchers to align their projects with these emerging companies, which is essential for accelerating research in Latin America and can potentially lead to fruitful partnerships and funding avenues. Moreover, the impact of Medtech clinical studies on local economies cannot be overlooked. These initiatives create jobs, stimulate economic growth, and improve healthcare outcomes, further underpinning the importance of collaboration in advancing global health.
Developing compelling grant proposals is essential. Proposals must include a detailed budget, restricted to a maximum of three pages, and clearly articulate objectives alongside potential impacts. This clarity will resonate with funding agencies that prioritize transparency and measurable outcomes.
Whitney Denning, manager of internationalization initiatives and events, emphasizes the importance of networking with funding agencies, stating,
For questions about this award, contact Whitney Denning, manager of internationalization initiatives and events.
Engaging with these agencies allows researchers to understand their specific priorities and application processes, which can significantly enhance the proposal's relevance. Researchers can effectively network by attending relevant conferences, participating in workshops, and utilizing platforms like LinkedIn to connect with agency representatives.
Additionally, a scatter plot illustrating the relationship between valuation and total funding for notable tech companies in the region could provide valuable insights into the funding landscape, highlighting the correlation between successful funding rounds and innovation potential.
Researchers should not overlook the potential of crowdfunding initiatives, which can effectively engage the public and raise awareness for their projects.
This modern approach not only diversifies funding strategies but also fosters community support and interest in scientific advancement. By adopting these strategies and leveraging the robust regulatory framework provided by INVIMA, researchers can significantly enhance their chances of accelerating research in Latin America and securing the resources necessary for successful projects in a rapidly evolving landscape.
Recruitment and Training: Building a Skilled Research Workforce
To develop a skilled inquiry workforce in South America, study leaders should implement various strategic measures. First, developing clear job descriptions that precisely outline the necessary skills and qualifications is essential for attracting the right candidates. Research indicates that targeted recruitment strategies can significantly enhance participation rates; for instance, the Latinx community accounted for 8.6%, 10.4%, and 13.7% of the populations in the three counties involved in recent recruitment efforts.
Descriptive statistics and logistic regression analyses conducted during this study further highlight the effectiveness of the recruitment strategies in increasing participant enrollment. Second, leveraging diverse recruitment channels—such as partnerships with universities, job fairs, and online platforms—can broaden the talent pool. Specifically, the partnership between bioaccess™ and Caribbean Health Group demonstrates a proactive strategy for accelerating research in Latin America by improving trial capabilities in Colombia and establishing Barranquilla as a prominent hub for studies, with support from Colombia's Minister of Health, who publicly backed the partnership during a meeting in Miami.
This support highlights the significance of governmental backing in attracting research trials to the region. Third, implementing comprehensive training programs that cover vital areas like methodologies, ethics, and compliance is crucial. As highlighted by the case study titled 'Addressing Barriers to Research Participation,' overcoming historical mistrust and ensuring effective navigation of healthcare settings can lead to increased engagement from underrepresented communities.
Furthermore, it is important to acknowledge that restrictive immigration laws may create a harsh environment for Latinx immigrants, further complicating their involvement in clinical studies. Lastly, promoting a collaborative atmosphere that highlights continuous learning and professional growth will not only enhance individual abilities but also contribute to overall quality. As Lopez-Cevallos & Harvey noted, the Latin America market is projected to grow significantly, with a market size of USD XX million in 2024 and a compound annual growth rate of 12.4% from 2024 to 2031.
This underscores the need for a skilled workforce to support the efforts of accelerating research in Latin America. Investing in human resources is essential for guaranteeing high-quality results and promoting innovation in healthcare studies. The collaboration's anticipated results comprise expanded clinical trial initiatives in Barranquilla, which will further strengthen its role as a center for medical studies in the region.
Future Trends: Innovations Shaping Research in Latin America
Anticipated trends are set to revolutionize research in Latin America, marking a transformative era characterized by several key developments that are crucial for accelerating research in Latin America:
- The integration of artificial intelligence and machine learning is becoming pivotal, enabling researchers to analyze vast datasets with unprecedented efficiency and refine study designs for enhanced outcomes. This technological shift is underscored by ongoing legislative efforts in Argentina to establish a robust framework for AI, including proposals that emphasize transparency, accountability, and human oversight. Nestor Maslej from the Stanford Institute for Human-Centered Artificial Intelligence emphasized, 'the broader the coverage, the more meaningful and impactful the results will be,' highlighting the importance of comprehensive regulation in yielding effective advancements.
- Moreover, Dr. Sergio Alvarado, a Clinical Trial Manager at bioaccess™, is at the forefront of this innovation, focusing on utilizing AI for diagnosing medical conditions and overseeing multiple projects that are aimed at accelerating research in Latin America to improve healthcare access.
- The expansion of telemedicine and remote monitoring technologies is facilitating greater patient engagement and more effective data collection. Recent statistics illustrate significant growth in telemedicine usage across the region, indicating a shift towards more accessible healthcare solutions.
- A focus on personalized medicine is emerging, driving research that caters to the unique needs of individual patients, underscoring the shift from one-size-fits-all approaches to tailored healthcare strategies. Collaborative platforms are gaining traction, fostering information sharing and innovation among researchers, which is essential for accelerating research in Latin America and advancing the field. The partnership between Greenlight Guru and bioaccess™ illustrates this trend, as they join forces to navigate the regulatory landscape and improve the quality of trials.
Additionally, Mexico's most relevant AI bill was submitted in April 2024, reflecting the region's commitment to establishing a regulatory framework. The SIRPLUX™ Drug-Coated Balloon, engineered for stent-like patency and restenosis prevention, represents a significant advancement in medical technology, offering higher potency with lower doses and ensuring nothing is left behind. Staying attuned to these trends is crucial for researchers aiming to excel in the dynamic landscape of Latin American clinical research, which is essential for accelerating research in Latin America.
Conclusion
The research landscape in Latin America presents both formidable challenges and promising opportunities. As highlighted throughout the article, institutions like INVIMA play a critical role in establishing a regulatory framework that supports innovation and clinical research. However, the region faces significant hurdles, including limited funding and bureaucratic complexities, which researchers must navigate effectively to advance their initiatives.
To enhance research capabilities, strategic investments in infrastructure, fostering collaborations, and encouraging interdisciplinary approaches are essential. By leveraging the unique advantages of the region—such as its diverse populations and emerging technologies—researchers can drive impactful clinical studies that not only address local health challenges but also contribute to global scientific knowledge.
Building strong partnerships across academia, industry, and government is vital for successful research outcomes. These collaborations can unlock new funding avenues and facilitate the sharing of expertise, ultimately driving innovation and improving healthcare delivery. Additionally, securing funding through various channels, including government grants and private sector partnerships, will be crucial for sustaining research efforts in an evolving economic landscape.
As the region moves forward, embracing trends such as artificial intelligence, telemedicine, and personalized medicine will be pivotal in shaping the future of research in Latin America. By remaining adaptable and proactive in addressing regulatory and funding challenges, researchers can position themselves at the forefront of scientific advancement, ensuring that Latin America becomes a leader in the global research arena.
Frequently Asked Questions
What is the role of INVIMA in Latin America's academic and research landscape?
INVIMA (Colombia National Food and Drug Surveillance Institute) is a critical player that oversees the marketing and manufacturing of health products, ensuring compliance with safety and efficacy standards. It monitors and controls medical devices and promotes clinical trials and innovation as a Level 4 health authority recognized by PAHO/WHO.
What challenges does Latin America face in accelerating research?
The region faces significant challenges such as limited funding, bureaucratic hurdles, and inconsistent regulatory frameworks. Additionally, interest payments in Latin America and the Caribbean have surpassed essential government expenditures, complicating the financial environment for scholarly initiatives.
How does the business sector contribute to research funding in Latin America?
In top R&D-performing countries, domestic businesses contribute at least 75% of funding for research and development, which is significantly higher compared to the U.S. (68%) and European countries (around 60%). This disparity highlights the importance of enhancing the business sector's role in financing studies in South America.
What are the strategic priorities for improving investigative abilities in South America?
Key strategic priorities include investing in modern development infrastructure, fostering collaborations between universities and industry, encouraging interdisciplinary studies, and implementing targeted training programs for researchers.
What advantages does Colombia offer for clinical trials?
Colombia offers competitive advantages such as cost efficiency, regulatory speed, high-quality healthcare, robust patient recruitment capabilities, and comprehensive clinical trial management services, making it a favorable destination for first-in-human clinical trials.
Why is interdisciplinary study important in addressing health issues in Latin America?
Interdisciplinary studies are crucial as they bring together diverse perspectives and expertise to create comprehensive solutions for complex health issues, thereby effectively tackling the challenges faced in the region.
What is the estimated investment needed for sustainable development goals in Latin America by 2030?
The region needs to invest approximately US$2,220,736 million across various sectors, including studies, to meet the Sustainable Development Goals (SDGs) by 2030.

