Overview
Effective project management for pivotal studies in Latin America can be achieved by implementing structured strategies such as clear objective setting, comprehensive planning, and stakeholder engagement. The article emphasizes that these practices not only streamline clinical research processes but also enhance collaboration and compliance, ultimately leading to improved patient outcomes and economic benefits in the region.
Introduction
In the dynamic landscape of clinical research, effective project management emerges as a cornerstone for success, particularly in regions like Latin America. Barranquilla, Colombia, is positioning itself as a pivotal hub for clinical trials through strategic collaborations aimed at overcoming regulatory hurdles and optimizing patient recruitment. The synergy between organizations such as bioaccess™ and Caribbean Health Group not only aims to enhance the local clinical research framework but also highlights the growing demand for skilled project managers in a field projected to expand significantly in the coming years.
As teams navigate complex approval processes and diverse healthcare systems, the implementation of robust project management practices becomes essential for ensuring compliance, fostering collaboration, and ultimately improving patient outcomes. This article delves into the critical strategies and insights necessary for navigating the multifaceted challenges of project management in clinical trials, emphasizing the profound impact these practices have on both healthcare advancements and local economies.
The Importance of Effective Project Management in Latin America
Efficient initiative oversight is vital in Latin America, especially in Barranquilla, where effective project management for pivotal studies in Latin America is being implemented by bioaccess™ and Caribbean Health Group to establish the city as a premier location for medical trials. This partnership, announced on March 29, 2019, during a meeting at PROCOLOMBIA's office in Miami, FL, is supported by Colombia's Minister of Health and aims to streamline the clinical research landscape, overcoming diverse regulatory environments, cultural nuances, and logistical challenges. With India anticipated to have 21.7 million job opportunities in overseeing initiatives by 2027, the need for qualified experts in this area is becoming more apparent.
Navigating the complex approval processes that vary significantly across countries is essential for conducting pivotal studies. Moreover, a deep understanding of local healthcare systems and patient demographics is vital for successful recruitment and retention of study participants. By adopting strong organizational practices, research teams can enhance collaboration, streamline workflows, and ultimately improve patient outcomes.
This strategic approach not only fosters compliance with varying regulations but also builds trust with stakeholders, which is essential for effective project management for pivotal studies in Latin America, ensuring that they are executed efficiently and ethically. Notably, GlobalCare Clinical Trials has partnered with bioaccess™ to enhance clinical trial ambulatory services in Colombia, achieving over a 50% reduction in recruitment time and 95% retention rates. As emphasized in the Horizon Databook, over 30% of our income is produced by collaborating with investment firms to pinpoint viable opportunity areas, highlighting the economic importance of efficient execution in the region.
Furthermore, the solution segment alone represented a revenue share of 66.58% in 2023, demonstrating the financial effect of effective oversight practices. A notable case study is the healthcare information system overhaul across several Latin American countries, illustrating the role of effective project management for pivotal studies in Latin America by demonstrating how a flexible system architecture can adapt to changes in government policy and local practices. This case demonstrates the beneficial effect of initiative oversight on local economies, including job creation and healthcare enhancement.
The increased use of management software due to digital advancements has significantly contributed to achieving smoother workflows and greater efficiency in management.
Step-by-Step Strategies for Successful Project Management
- Define Clear Objectives: Begin by setting specific, measurable, achievable, relevant, and time-bound (SMART) objectives for your research trial. This clarity not only guides your initiative but also aligns team efforts towards a common goal. Experts emphasize that clearly defined objectives are crucial for success, particularly in the complex landscape of clinical research. Notably, 29% of organizations mostly or always finish tasks on time, underscoring the significance of effective objective setting in achieving timely outcomes.
- Develop a Comprehensive Plan: Craft a detailed plan that outlines timelines, milestones, and resource allocations. It is also essential to include contingency plans to manage potential risks effectively. A robust plan serves as a blueprint for navigating the intricacies of research trials.
- Assemble a Competent Team: Recruit skilled individuals who possess the necessary expertise to fulfill roles. Encourage collaboration and foster open communication among team members to create a supportive environment that enhances productivity and innovation.
- Implement Regular Monitoring and Evaluation: Establish key performance indicators (KPIs) to monitor progress throughout the study. Regularly reviewing these metrics allows for the identification of areas needing improvement, leading to timely adjustments in strategies. Recent regression analyses have indicated that quality and speed directly relate to overall healthcare study success, emphasizing the significance of monitoring and assessment in achieving objectives.
- Cultivate Stakeholder Relationships: Actively engage with stakeholders, including regulatory bodies, ethics committees, and patient advocacy groups. Establishing strong connections is essential for enabling smoother approvals and boosting trust in your endeavor, thereby aiding its success.
- Ensure Compliance with Regulations: Stay abreast of local regulations and ethical guidelines relevant to clinical trials. Conduct training sessions for your team to ensure everyone is well-informed about compliance requirements, reinforcing the importance of adhering to established standards. This includes detailed processes for compliance reviews, ensuring all documentation meets regulatory expectations.
- Leverage Technology for Efficiency: Utilize task coordination software to streamline communication, documentation, and tracking processes. This technology can enhance collaboration and provide real-time updates on the status, ultimately improving overall efficiency.
- Participate in Extensive Clinical Study Oversight Services: Our service abilities include feasibility assessments, site selection, compliance evaluations, study setup and approvals, import permits, oversight, and thorough reporting on study status and adverse events. By leveraging these services, research teams can ensure thorough oversight and execution of their studies, contributing to advancements in medical knowledge and improved patient outcomes.
By following these step-by-step strategies, research teams can ensure effective project management for pivotal studies in Latin America, thereby contributing to advancements in medical knowledge and improved patient outcomes. Furthermore, with the project management workforce projected to grow by 33% across 11 countries by 2027, the demand for adept project managers emphasizes the critical need for effective project management for pivotal studies in Latin America.
Navigating Regulatory Challenges
Navigating the regulatory landscape in Latin America, particularly in Colombia, necessitates a comprehensive understanding of both local and international guidelines. Colombia exemplifies effective project management for pivotal studies in Latin America, especially first-in-human (FIH) studies, due to its cost efficiency, achieving savings of over 30% compared to studies in North America and Western Europe. The regulatory speed is notable, with IRB/EC and INVIMA approvals typically taking only 90-120 days.
Additionally, Colombia's healthcare system ranks among the best globally, ensuring high-quality patient care and recruitment from a population of over 50 million, with about 95% having universal healthcare coverage. Furthermore, R&D tax incentives provide significant financial benefits, including a 100% tax deduction and various credits, contributing to effective project management for pivotal studies in Latin America, making Colombia an even more attractive option for clinical trials.
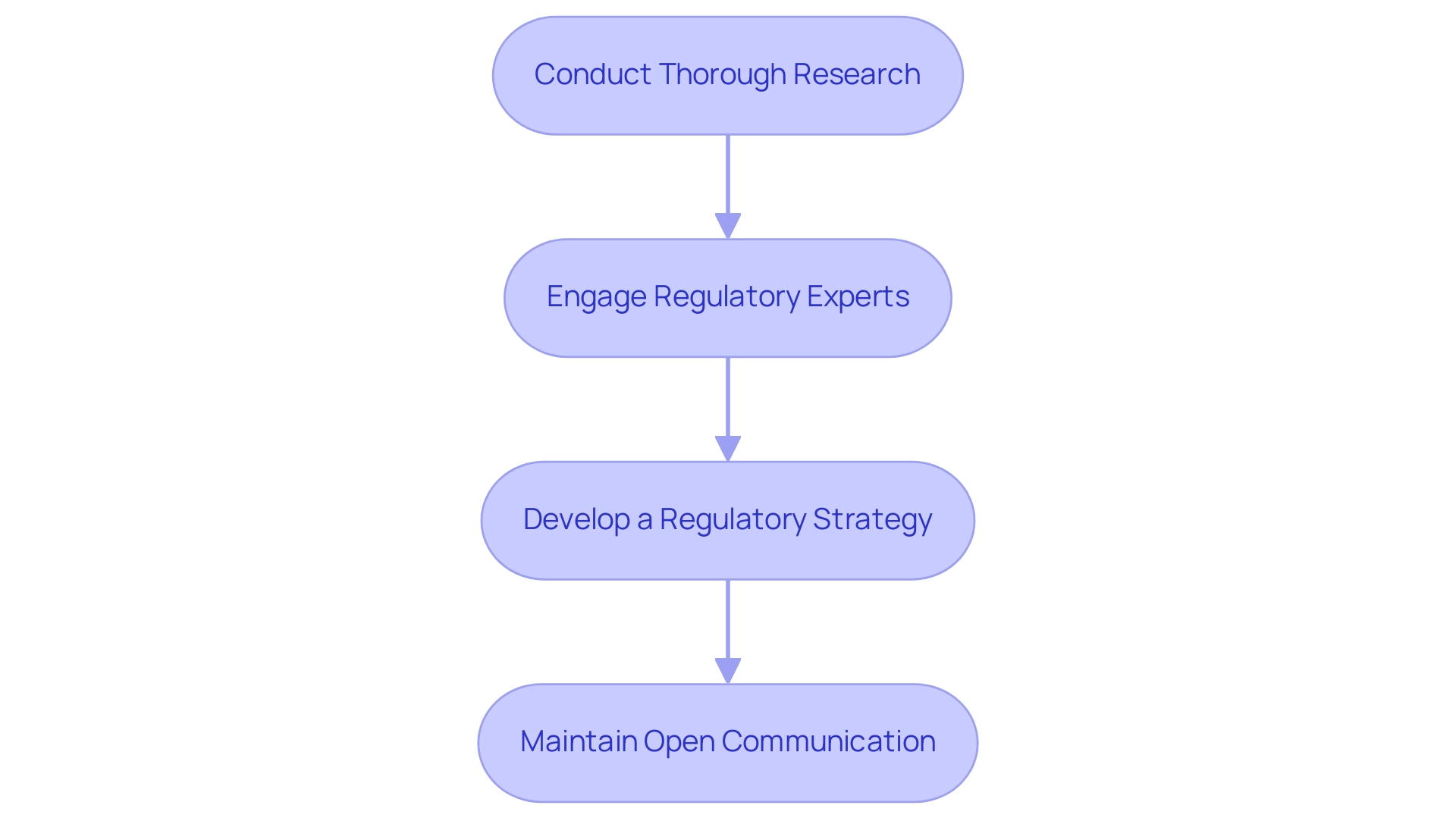
- Conduct Thorough Research: Gain familiarity with the specific regulatory requirements in Colombia, leveraging resources from local regulatory authorities and industry associations to stay informed of any updates or changes.
- Engage Regulatory Experts: Enlisting the assistance of Regulatory Affairs specialists, such as Ana Criado, Director of Regulatory Affairs at Mahu Pharma, and Katherine Ruiz, an expert in Regulatory Affairs for Medical Devices, can significantly streamline the approval process. Their expertise ensures effective project management for pivotal studies in Latin America while adhering to local mandates and enhancing the overall efficiency of management. These experts can also assist with feasibility studies and compliance reviews, which are crucial for successful execution.
- Develop a Regulatory Strategy: Formulate a comprehensive regulatory strategy that outlines the essential steps for obtaining approvals and ensuring compliance throughout the study's duration. This strategy should detail timelines, responsible parties, and potential pitfalls to avoid for effective project management for pivotal studies in Latin America.
- Maintain Open Communication: Establish and nurture relationships with regulatory bodies by promoting open communication. Regular updates and transparency can lead to smoother interactions and expedite the approval process. This is especially crucial considering the expansion of medical research in Colombia and the wider Andean area, emphasizing the rising significance of customized regulatory approaches.
Moreover, funding in the trial sector in Colombia has escalated, indicating the country's increasing involvement in FDA-regulated studies and the internationalization of medical research, highlighting the need for a strong regulatory strategy to support successful trials in the area.
Effective Communication and Team Collaboration
Effective communication and teamwork among group members are essential elements for successful execution in clinical research. To bolster these aspects, consider the following strategies:
-
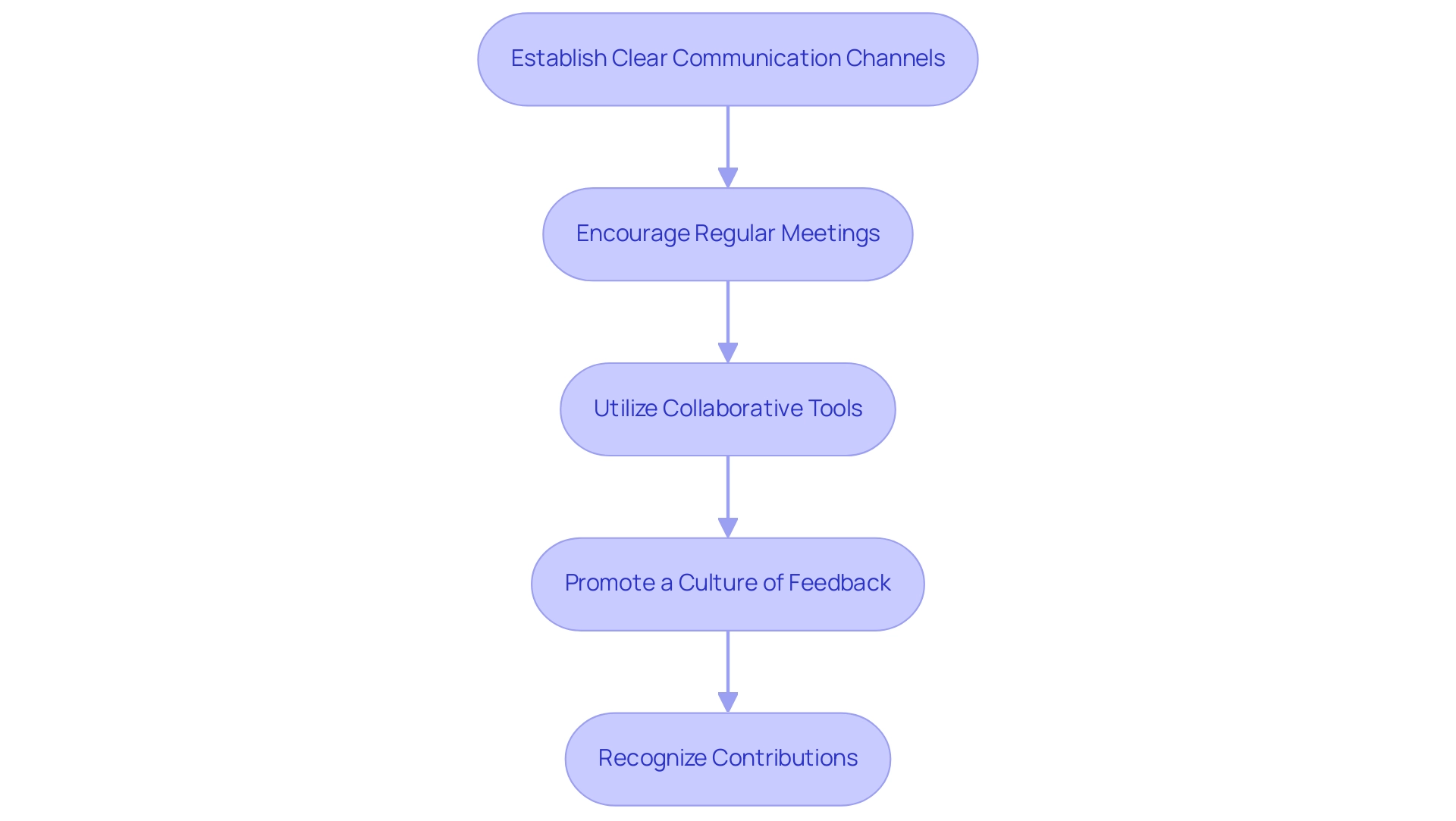
Establish Clear Communication Channels: Define and convey the preferred methods for team interactions, whether through emails, meetings, or specialized tools for collaboration.
Ensuring that all members understand these channels promotes efficiency and reduces misunderstandings.
-
Encourage Regular Meetings: Schedule consistent team meetings to review progress, tackle challenges, and share updates. This practice not only fosters accountability but also aligns all team members with the overarching project goals, enhancing collective focus.
-
Utilize Collaborative Tools: Adopt advanced collaborative software, such as Slack or Trello, to facilitate seamless communication and task organization. Research indicates that 74% of businesses have implemented new tools to optimize organization and administration due to the rise of remote and hybrid work environments, underscoring the importance of leveraging technology to enhance team collaboration.
-
Promote a Culture of Feedback: Cultivate an environment where team members feel encouraged to provide constructive feedback and share their ideas openly.
A culture grounded in communication can lead to innovative solutions, enhance team morale, and ultimately drive success. Notably, 40% of individuals reported improved communication within their workplaces over the past year, highlighting the positive impact of fostering open dialogue.
-
Recognize Contributions: Acknowledge and celebrate individual and team achievements, regardless of their scale.
Acknowledging contributions not only inspires team members but also promotes a feeling of inclusion and dedication to the initiative.
By emphasizing these effective communication and collaboration techniques, research teams can significantly improve their ability to handle tasks, which is essential for effective project management for pivotal studies in Latin America. According to a recent report, 82% of organizations utilizing planning tools evaluate their performance as 'excellent' or 'above average', illustrating the tangible benefits of structured communication and collaboration. Furthermore, insights from the case study titled IPM's Quarterly Digest: Achieving Project Success with Problem-Solving, Leadership, and Program Management (Vol. 2) emphasize essential strategies that aid in efficient execution and success, underscoring the significance of these practices in medical research.
Risk Management and Contingency Planning
Effective project management for pivotal studies in Latin America is crucial, especially as research extends into various areas, highlighting the importance of risk management. Collaborations, such as that between bioaccess™ and Caribbean Health Group, underscore the importance of positioning Barranquilla as a leading destination for clinical trials, backed by the support of Colombia's Minister of Health, Juan Pablo Uribe. To adeptly identify and mitigate risks, consider the following steps:
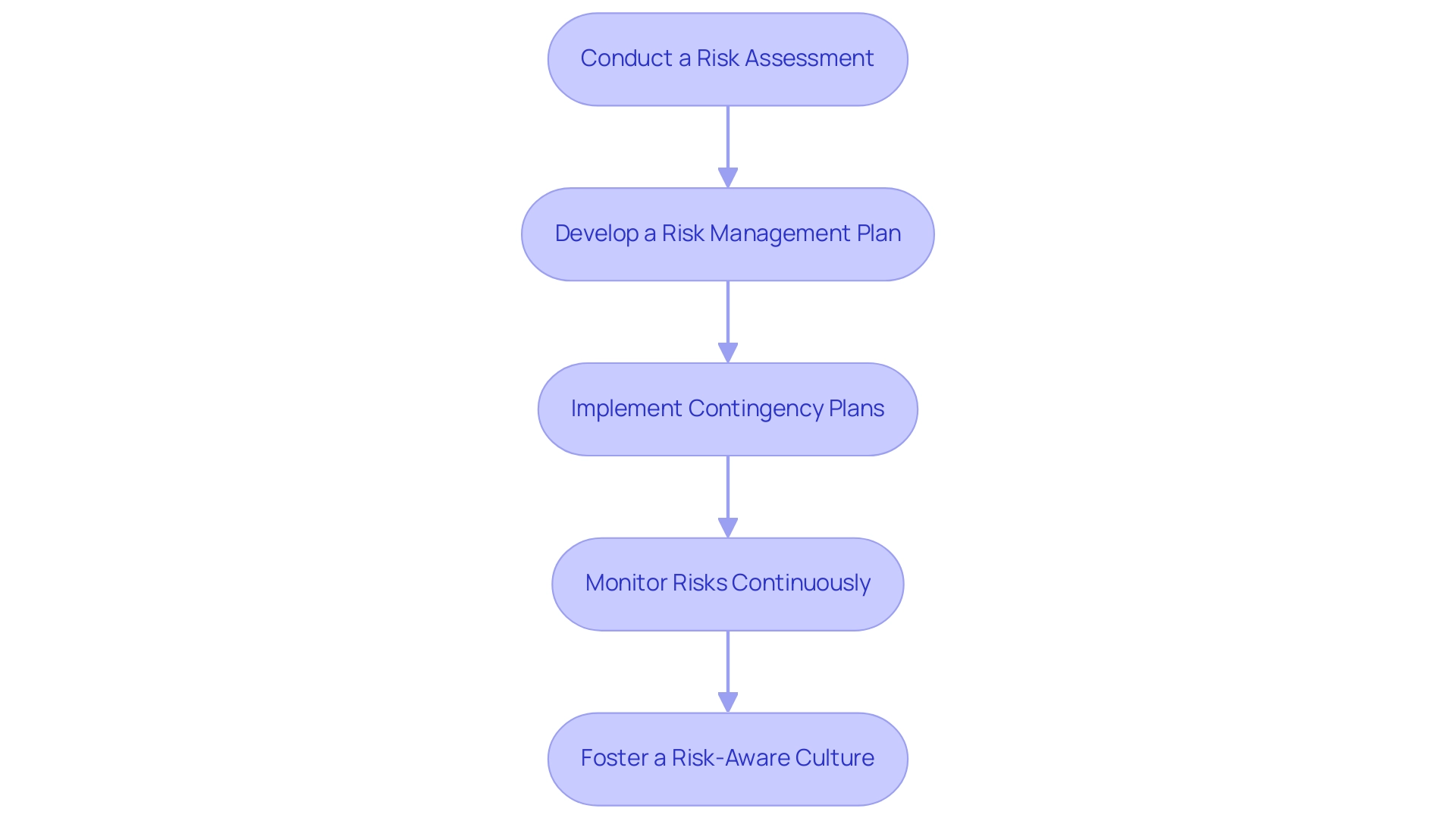
- Conduct a Risk Assessment: Begin by pinpointing potential risks that may affect the study, such as regulatory changes, recruitment challenges, or budget constraints. Assess the likelihood and impact of each risk to prioritize them effectively.
- Develop a Risk Management Plan: Formulate a comprehensive plan that outlines specific strategies for mitigating the identified risks. Assign clear responsibilities for monitoring and addressing each risk, ensuring accountability within the team.
- Implement Contingency Plans: Design contingency plans for the most significant risks. These plans should articulate alternative strategies to be employed if initial plans encounter obstacles, fostering resilience in the project.
- Monitor Risks Continuously: Engage in regular reviews and updates of the risk control plan throughout the study's duration. This proactive approach facilitates timely adjustments, minimizing disruptions and maintaining momentum.
- Foster a Risk-Aware Culture: Cultivate an environment where team members are encouraged to remain vigilant about potential risks and to communicate concerns without hesitation. A culture prioritizing risk awareness can significantly improve the handling of unforeseen challenges.
Statistics indicate that approximately 70% of research projects struggle with inadequate risk oversight, underscoring the necessity for structured approaches. Sean Salleh, a data scientist with a master's degree in Operations Research, emphasizes that effective forecasting and scenario planning are vital in navigating these complexities. The methods examined in the 2015 J Risk Res publication emphasize the significance of customized risk strategies that can adjust to the distinct challenges of medical studies.
Moreover, as upcoming research in various nations arises, they could offer insights into the issues of trial locations concerning performance and risk approaches, strengthening the necessity for flexible strategies across different areas. Furthermore, case studies have demonstrated that effective data handling practices, which comply with privacy laws while maintaining data integrity, are essential for informed decision-making in research involving patients. By integrating strong risk oversight and contingency planning, research teams can maneuver through the intricacies of trials with enhanced confidence and resilience, ultimately aiding in the economic growth and healthcare enhancement in local communities.
This collaboration also emphasizes essential services such as feasibility studies, site selection, compliance reviews, trial setup, import permits, and effective project management for pivotal studies in Latin America, all of which are critical for successful clinical trials in Barranquilla.
Conclusion
The effective management of clinical trials in Latin America, particularly in Barranquilla, Colombia, is pivotal for advancing healthcare and fostering economic growth. As organizations like bioaccess™ and Caribbean Health Group collaborate to streamline the clinical research landscape, the importance of skilled project managers becomes increasingly evident. The article highlights that robust project management practices are essential for navigating complex regulatory environments and optimizing patient recruitment, ultimately leading to improved patient outcomes.
Key strategies for successful project management have been outlined, including:
- Establishment of clear objectives
- Comprehensive planning
- Effective communication among team members
By leveraging technology and fostering strong stakeholder relationships, research teams can enhance collaboration and ensure compliance with local regulations. Furthermore, the significance of risk management and contingency planning cannot be overstated, as these practices are critical for mitigating potential challenges and maintaining project momentum.
In conclusion, the burgeoning demand for clinical trials in regions like Barranquilla underscores the necessity for adept project management. As the field continues to evolve, prioritizing these strategies will not only contribute to advancements in medical knowledge but also bolster local economies. The synergy between effective project management and clinical research will play a crucial role in shaping the future of healthcare in Latin America, making it an exciting area for both investment and innovation.
Frequently Asked Questions
Why is efficient initiative oversight important in Latin America, specifically in Barranquilla?
Efficient initiative oversight is vital in Latin America, particularly in Barranquilla, to establish the city as a premier location for medical trials. It helps streamline the clinical research landscape and addresses regulatory, cultural, and logistical challenges.
What partnership was announced to improve project management for clinical studies in Barranquilla?
A partnership between bioaccess™ and Caribbean Health Group was announced on March 29, 2019, to enhance project management for pivotal studies in Barranquilla, supported by Colombia's Minister of Health.
What are the key challenges faced in conducting pivotal studies in Latin America?
Key challenges include navigating complex approval processes that vary across countries, understanding local healthcare systems, and addressing patient demographics for successful recruitment and retention of study participants.
How can research teams improve collaboration and patient outcomes?
By adopting strong organizational practices, research teams can enhance collaboration, streamline workflows, and ultimately improve patient outcomes.
What impact does effective project management have on local economies in Latin America?
Effective project management can lead to job creation and healthcare enhancement, as demonstrated by case studies like the healthcare information system overhaul across several Latin American countries.
What role does management software play in project management for clinical trials?
The increased use of management software has significantly contributed to smoother workflows and greater efficiency in project management.
What are SMART objectives, and why are they important?
SMART objectives are specific, measurable, achievable, relevant, and time-bound goals that guide research trials. They are crucial for aligning team efforts and achieving timely outcomes.
What are some key strategies for effective project management in clinical studies?
Key strategies include developing a comprehensive plan, assembling a competent team, implementing regular monitoring and evaluation, cultivating stakeholder relationships, ensuring compliance with regulations, leveraging technology for efficiency, and participating in extensive clinical study oversight services.
What is the projected growth for the project management workforce in Latin America?
The project management workforce is projected to grow by 33% across 11 countries by 2027, highlighting the critical need for effective project management in the region.




