Overview
Addressing cultural sensitivities in multinational clinical trials is crucial for ensuring diverse representation and improving participant engagement, which ultimately enhances the validity of research findings. The article emphasizes that strategies such as engaging local communities, tailoring recruitment materials, and providing cultural competency training for research staff are essential steps to foster trust and inclusivity, thereby mitigating historical gaps in representation among various populations.
Introduction
In the realm of clinical research, the significance of cultural nuances cannot be overstated, particularly in multinational clinical trials where diverse populations are involved. Understanding the beliefs, values, and customs that shape participant behavior is essential for ensuring equitable representation and successful outcomes.
Historical disparities in clinical trial participation, especially among racial and ethnic minorities, highlight the pressing need for researchers to address cultural sensitivities and engage with communities effectively. By employing targeted strategies that enhance cultural responsiveness, researchers can not only improve recruitment and retention rates but also foster trust and integrity throughout the research process.
This article delves into the multifaceted role of culture in clinical trials, exploring effective methodologies for enhancing diversity, ethical considerations, and the importance of language and communication in achieving successful research outcomes.
Understanding Cultural Nuances in Multinational Clinical Trials
Cultural nuances play a pivotal role in shaping the beliefs, values, customs, and behaviors that vary across populations. In the context of multinational clinical studies, addressing cultural sensitivities in multinational clinical trials is essential for researchers to acknowledge how these cultural factors influence participant perceptions and responses. For instance, during the Moderna Covid-19 vaccine study, Black Americans represented only 7% of the study population, despite accounting for 13% of the U.S. population, highlighting a gap in representation that can stem from cultural beliefs regarding medical interventions and trust in healthcare systems.
To mitigate such challenges, conducting thorough cultural assessments prior to initiating studies is crucial. These assessments can unveil specific cultural perspectives that may affect participation and adherence. Engaging local experts or cultural consultants not only enriches the team's understanding of the target population but also enhances the design and implementation of the study.
The NIH highlights the necessity for this approach, indicating that their 2021–2025 strategic plan to promote studies on the health and well-being of sexual and gender minority (SGM) populations reveals that SGM represent a health-disparities group and suggests offering assistance for new investigators to establish a robust SGM workforce, while enhancing projects connected to SGM health.
The report on Historical Exclusion from Medical Studies discusses the historical background of the exclusion of women and minorities from medical studies, particularly following past tragedies linked to drug testing. Persistent gaps in representation, particularly for pregnant women and racial/ethnic minorities, continue to challenge health equity. To enhance results and guarantee that research studies represent the diversity of the populations they intend to assist, addressing cultural sensitivities in multinational clinical trials is essential to consider at every phase of the research process.
This may include:
- Designing culturally appropriate recruitment strategies
- Providing materials in multiple languages
- Ensuring that protocols are sensitive to cultural practices and beliefs
Strategies for Enhancing Cultural Responsiveness in Clinical Trials
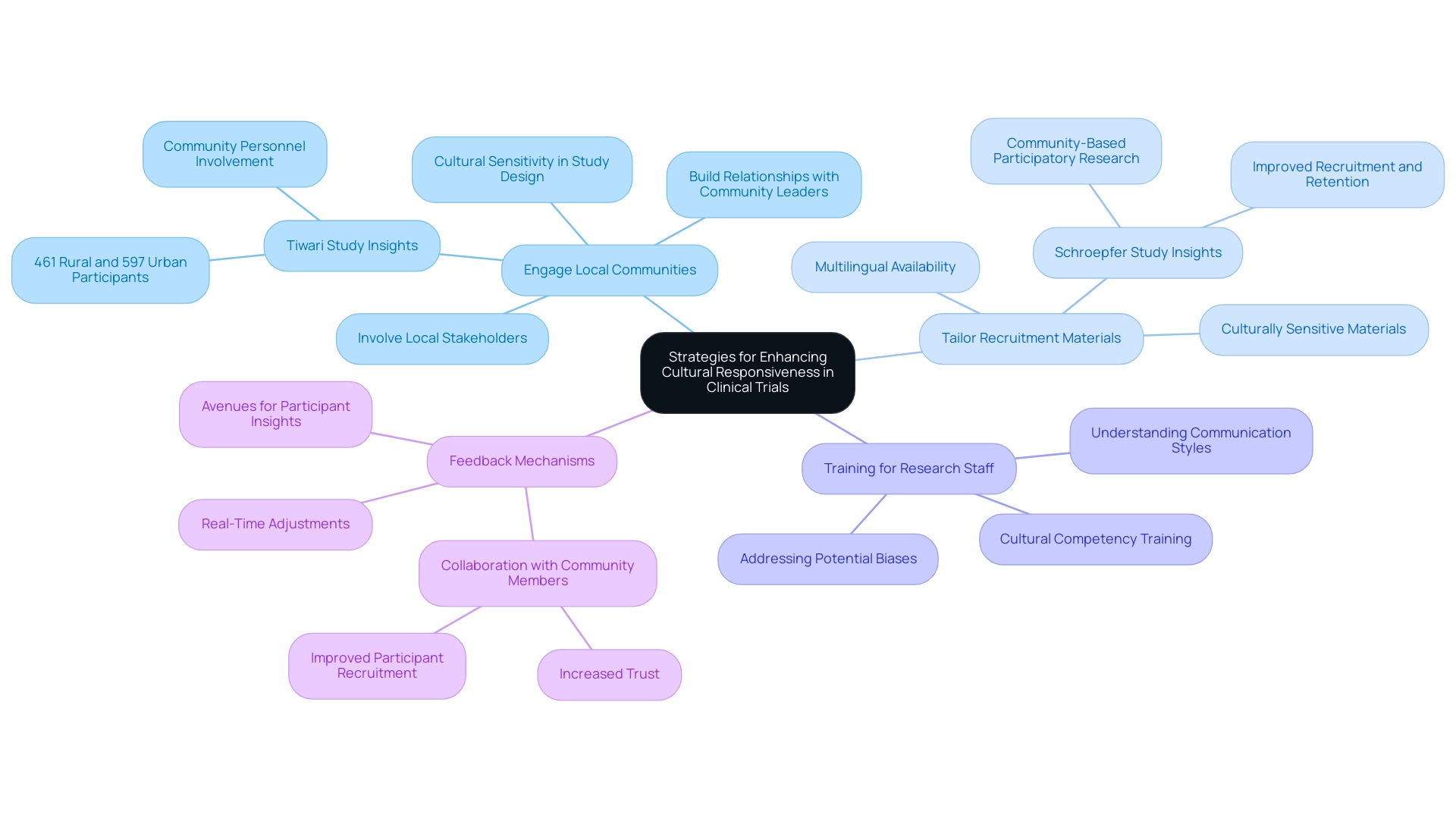
- Engage Local Communities: Establishing strong relationships with community leaders and organizations is crucial for fostering trust and encouraging participation in clinical trials. Involving local stakeholders during the planning stages is crucial for addressing cultural sensitivities in multinational clinical trials, leading to culturally relevant study designs that resonate with the community. A study emphasized by Tiwari in 2014, which included 461 rural and 597 urban individuals, underscores the effectiveness of involving community personnel to enhance recruitment and build trust. However, it is important to note that co-leading with community personnel was a principle in only 2 studies, indicating a need for greater involvement in future investigations.
- Tailor Recruitment Materials: It is essential to ensure that all recruitment materials, including consent forms and informational brochures, are culturally sensitive, which is a key aspect of addressing cultural sensitivities in multinational clinical trials, and available in multiple languages. This approach not only acknowledges the cultural backgrounds of individuals but also plays a crucial role in addressing cultural sensitivities in multinational clinical trials, helping them feel valued and respected. Case studies, like Schroepfer's 2009 research involving rural American Indian individuals, demonstrate that tailored materials significantly enhance recruitment and retention rates. Specifically, this study showed that using culturally appropriate materials led to a higher engagement level among those involved.
- Training for Research Staff: Offering cultural competency training for all team members is essential for addressing cultural sensitivities in multinational clinical trials and improving their comprehension of the cultural contexts of those involved. This training should encompass communication styles, cultural norms, and potential biases. Such preparation equips personnel to interact effectively with diverse populations, which is increasingly important for addressing cultural sensitivities in multinational clinical trials.
- Feedback Mechanisms: Creating avenues for individuals to share their insights regarding their experiences during the study is essential. This information can be invaluable for addressing cultural sensitivities in multinational clinical trials, allowing for real-time adjustments to improve cultural responsiveness. Recent findings indicate that addressing cultural sensitivities in multinational clinical trials through collaboration with community members, as reported in 15 articles, has led to increased trust and improved participant recruitment, further emphasizing the necessity of continuous engagement and feedback in health studies. As Suzanne Day from the Institute for Global Health and Infectious Diseases states, "This experience emphasizes the significance of viewing community engagement as a continuous process of establishing sustainable relationships between researchers and the local populations in which studies are integrated.
The Role of Diversity in Clinical Trial Success
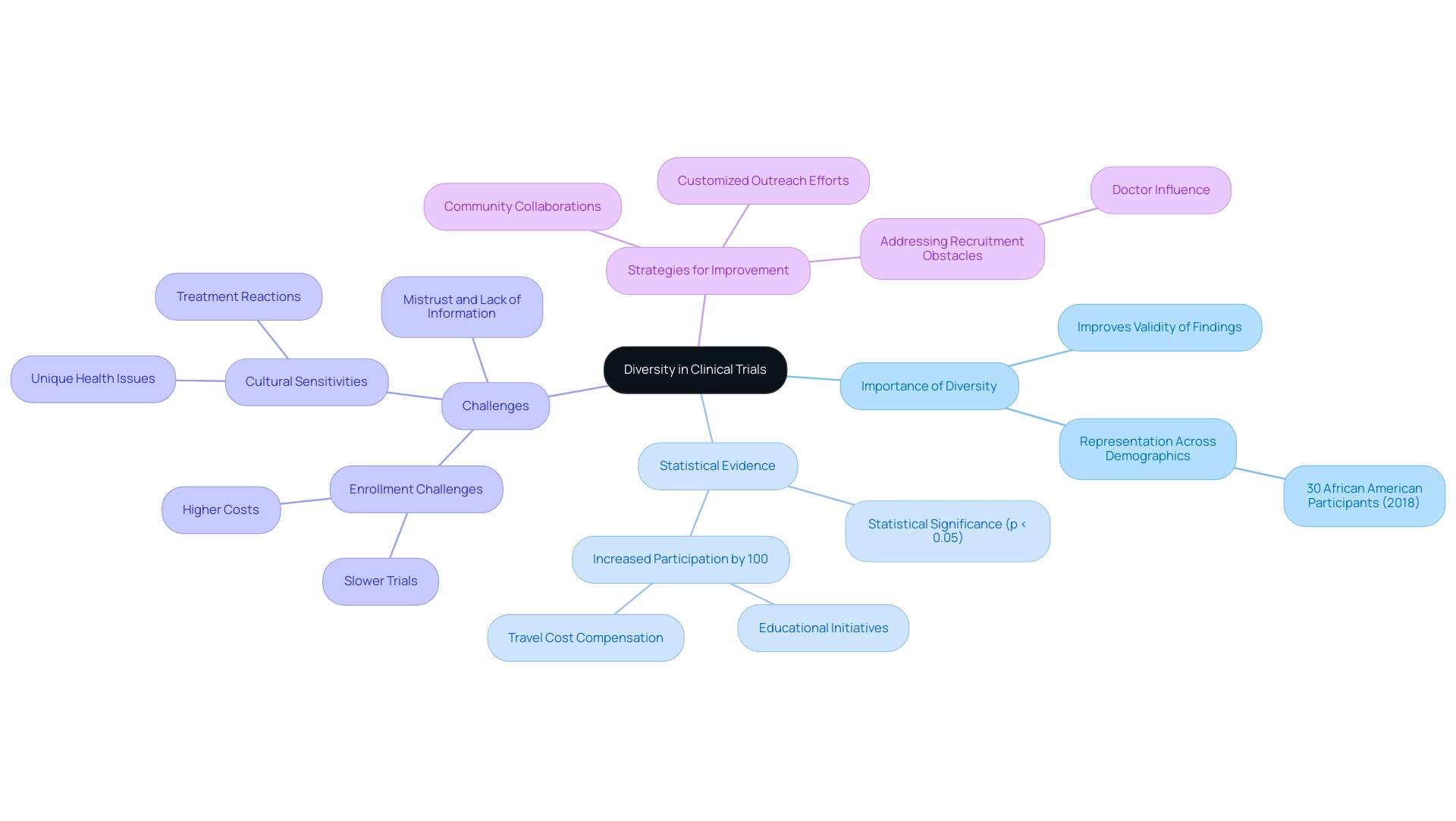
Addressing cultural sensitivities in multinational clinical trials through the incorporation of diversity in medical studies significantly improves the validity and relevance of findings across different populations. As emphasized in recent statistics, in 2018, over 30% of research subjects identified as African American or Black, indicating a growing acknowledgment of the need for diverse representation. However, another study of key barriers in Alzheimer’s disease exploration has indicated that enrollment challenges result in slower and more expensive trials, suggesting the need for nationally coordinated efforts to increase diverse participation in clinical trials in this and other disease areas (Malzbender et al.).
To further this objective, researchers must actively pursue participants from a wide range of demographic backgrounds, encompassing various ethnicities, genders, and socioeconomic statuses. Strategic outreach efforts, including collaborations with community organizations that cater to underrepresented groups, can facilitate this process. For example, a study addressing barriers to participation in Alzheimer’s disease research revealed that educational initiatives and travel cost compensation led to a remarkable increase of over 100% in Black patient participation.
This illustrates that customized outreach can effectively involve varied groups and increase the significance of research studies. Additionally, it is essential to recognize that respondents identifying as non-Hispanic Black had lower odds of being influenced 'A lot' by their doctor promoting involvement in research studies compared to non-Hispanic White respondents, emphasizing a significant obstacle to recruitment that needs to be tackled. Furthermore, addressing cultural sensitivities in multinational clinical trials is essential, as research designs must consider the unique health issues and treatment reactions common among various groups, ensuring that the studies are not only inclusive but also relevant to all participants.
Such factors are crucial for the success of research studies advancing into 2024 and beyond, as they ultimately lead to more robust and generalizable findings. Statistical significance was established at p < 0.05, further highlighting the importance of these analyses in understanding diversity in medical studies.
Ethical Considerations in Addressing Cultural Sensitivities
In multinational studies, researchers must navigate significant ethical challenges, particularly regarding informed consent and the risk of coercion. To ensure the well-being of participants, it is essential to provide full information about the study's objectives, procedures, and potential risks, particularly by addressing cultural sensitivities in multinational clinical trials. As Professor Alex John London articulates, 'When you ensure that the information is relevant to the local population, you build the foundation of knowledge necessary to generate beneficial interventions and policies, and this is a kind of benefit itself.'
This underscores the necessity of clear communication tailored to diverse cultural contexts, particularly in addressing cultural sensitivities in multinational clinical trials. To tackle these challenges effectively, comprehensive research management services play a vital role, encompassing:
- Feasibility studies
- Site selection
- Compliance reviews
- Study setup
- Import permits
- Project management
- Reporting
Specifically, the setup process involves designing protocols, obtaining necessary approvals, and training site staff to ensure adherence to regulatory standards.
These services not only enhance the ethical governance of research trials but also contribute to local economies through:
- Job creation
- Economic growth
- Healthcare improvements
- International collaboration
Moreover, the CTSA consortium's definition of core competencies for masters-level translational investigators emphasizes the significance of ethical factors in medical studies. Scientists should stay sharply aware of existing power dynamics between the investigation group and study subjects, particularly within vulnerable populations.
Creating an ethics review board made up of varied members can guarantee that ethical considerations are fully incorporated throughout the research process, ultimately promoting trust and integrity in multinational studies. Collaboration among statisticians, scientists, and other stakeholders is also crucial for the accurate interpretation of statistical results, as demonstrated in the case study titled 'Interpretable Quantification of Evidence,' which illustrates the necessity of cautious interpretation, particularly regarding confidence intervals and the potential for biased estimates in genomic studies.
The Importance of Language and Communication in Multinational Trials
Language obstacles present considerable difficulties in recruiting and retaining individuals within research studies. Research indicates that individuals with Limited English Proficiency (LEP) often feel the need to be particularly careful about their appearance to ensure fair treatment. In fact, 48% of adults with LEP report this concern, highlighting the need for sensitive engagement strategies.
Furthermore, Wilson et al. found that 49% of LEPPs struggle to understand medical situations, which underscores the critical nature of effective communication strategies in clinical trials. To tackle these challenges, it is essential for researchers to employ professional translation services for all study materials, ensuring clarity and accessibility for those involved.
Additionally, hiring bilingual staff can greatly enhance communication, fostering a more inclusive environment. It is equally important to acknowledge that communication styles can vary significantly across cultures; thus, addressing cultural sensitivities in multinational clinical trials by training researchers to adapt their approaches accordingly is essential. Incorporating visual aids and culturally relevant examples can further enhance understanding and engagement.
Regular assessment of communication strategies is essential, as it enables researchers to pinpoint areas for enhancement and ensures that all individuals feel included and informed. A relevant case study demonstrated that utilizing speech-enabled phraselators in emergency settings improved healthcare accessibility for patients facing language barriers, illustrating the potential benefits of effective communication strategies in clinical settings. Moreover, as noted by Hanvey GA, addressing cultural sensitivities in multinational clinical trials through integrated interventions can significantly enhance engagement and overall trial success.
Additionally, Nguyen et al.'s pilot study on Asian American women's participation in cancer treatment research offers insights into the importance of cultural considerations in participant engagement.
Conclusion
Cultural nuances are integral to the success of multinational clinical trials, influencing not only participant recruitment and retention but also the overall integrity of research outcomes. As demonstrated throughout the article, acknowledging and addressing historical disparities in clinical trial participation is paramount. By implementing culturally sensitive methodologies—such as engaging local communities, tailoring recruitment materials, and providing comprehensive training for research staff—researchers can enhance trust and foster a more inclusive environment.
Moreover, the role of diversity in clinical trials cannot be overstated. Diverse representation not only enriches the research landscape but also ensures that findings are applicable across various populations. The necessity for ethical considerations in navigating cultural sensitivities further underscores the responsibility researchers hold in conducting equitable research. This includes transparent communication and informed consent processes that respect the cultural contexts of all participants.
Ultimately, the commitment to cultural responsiveness in clinical trials is not just a best practice; it is essential for achieving equitable health outcomes. By prioritizing these strategies, researchers can contribute to a more inclusive and effective clinical research environment, paving the way for advancements that benefit all segments of society. As the field continues to evolve, embracing cultural diversity will remain a cornerstone for future success in clinical research.
Frequently Asked Questions
Why are cultural nuances important in multinational clinical studies?
Cultural nuances shape beliefs, values, customs, and behaviors that vary across populations, influencing participant perceptions and responses in clinical trials.
What was a notable example highlighting the gap in representation in clinical studies?
During the Moderna Covid-19 vaccine study, Black Americans represented only 7% of the study population, despite accounting for 13% of the U.S. population, indicating a gap in representation influenced by cultural beliefs and trust in healthcare systems.
How can researchers address cultural sensitivities in clinical trials?
Researchers can conduct thorough cultural assessments prior to studies, engage local experts or cultural consultants, and design culturally appropriate recruitment strategies to better understand and respect the target population's perspectives.
What does the NIH's strategic plan emphasize regarding SGM populations?
The NIH's 2021–2025 strategic plan promotes studies on the health and well-being of sexual and gender minority (SGM) populations, recognizing them as a health-disparities group and suggesting support for establishing a robust SGM workforce.
What historical issues have contributed to gaps in representation in medical studies?
Historical exclusion of women and minorities from medical studies, particularly following past tragedies linked to drug testing, has resulted in persistent gaps in representation, especially for pregnant women and racial/ethnic minorities.
What are some strategies to enhance cultural sensitivity in clinical trials?
Strategies include designing culturally appropriate recruitment strategies, providing materials in multiple languages, and ensuring protocols are sensitive to cultural practices and beliefs.
Why is engaging local communities important in clinical trials?
Engaging local communities fosters trust and encourages participation, leading to culturally relevant study designs that resonate with the community.
How can recruitment materials be tailored to address cultural sensitivities?
Recruitment materials, including consent forms and brochures, should be culturally sensitive and available in multiple languages to acknowledge the backgrounds of individuals and help them feel valued.
What is the significance of training for research staff in cultural competency?
Training equips research staff with the understanding of communication styles, cultural norms, and potential biases, enabling them to interact effectively with diverse populations.
How can feedback mechanisms improve cultural responsiveness in clinical trials?
Creating avenues for participants to share their experiences allows for real-time adjustments to improve cultural responsiveness, enhancing trust and recruitment in health studies.

