Introduction
In the competitive landscape of clinical research for medical devices, the selection of a Contract Research Organization (CRO) is a critical decision that can significantly impact both the efficiency and cost-effectiveness of a study. With the rise of Medtech startups in Latin America, organizations are increasingly turning to specialized CROs like bioaccess® to navigate the complexities of clinical trials while maximizing their budgets. This article delves into the essential strategies for choosing a cost-effective CRO, emphasizing the importance of balancing financial considerations with the quality of services provided.
By understanding the key factors involved in this selection process, stakeholders can ensure that their investments yield optimal results and contribute to the advancement of healthcare innovation.
Understanding the Importance of Cost-Effectiveness in CRO Selection
In the realm of clinical studies for medical devices, selecting a cost-effective Contract Research Organization (CRO) like bioaccess® is paramount. As a US-based CRO, bioaccess® specializes in facilitating accelerated clinical services for Medtech startups in Latin America, providing fast, cost-effective, and high-quality clinical data. The appropriate CRO can significantly affect the overall budget, impacting both timelines and outcomes. Organizations must concentrate on cost-effectiveness to guarantee that funds are distributed wisely, enabling the possibility to reinvest savings into other essential fields of study or development.
While lower costs are attractive—often exceeding 30% savings compared to trials in North America or Western Europe—these should not come at the expense of quality and compliance. Colombia's competitive advantages, including regulatory speed, high-quality healthcare, and robust patient recruitment, make it an ideal destination for first-in-human clinical trials.
bioaccess® provides an extensive array of services, such as:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Project management
ensuring that high standards are upheld while attaining the best financial results.
Key Strategies for Choosing a Cost-Effective CRO for Medical Devices
-
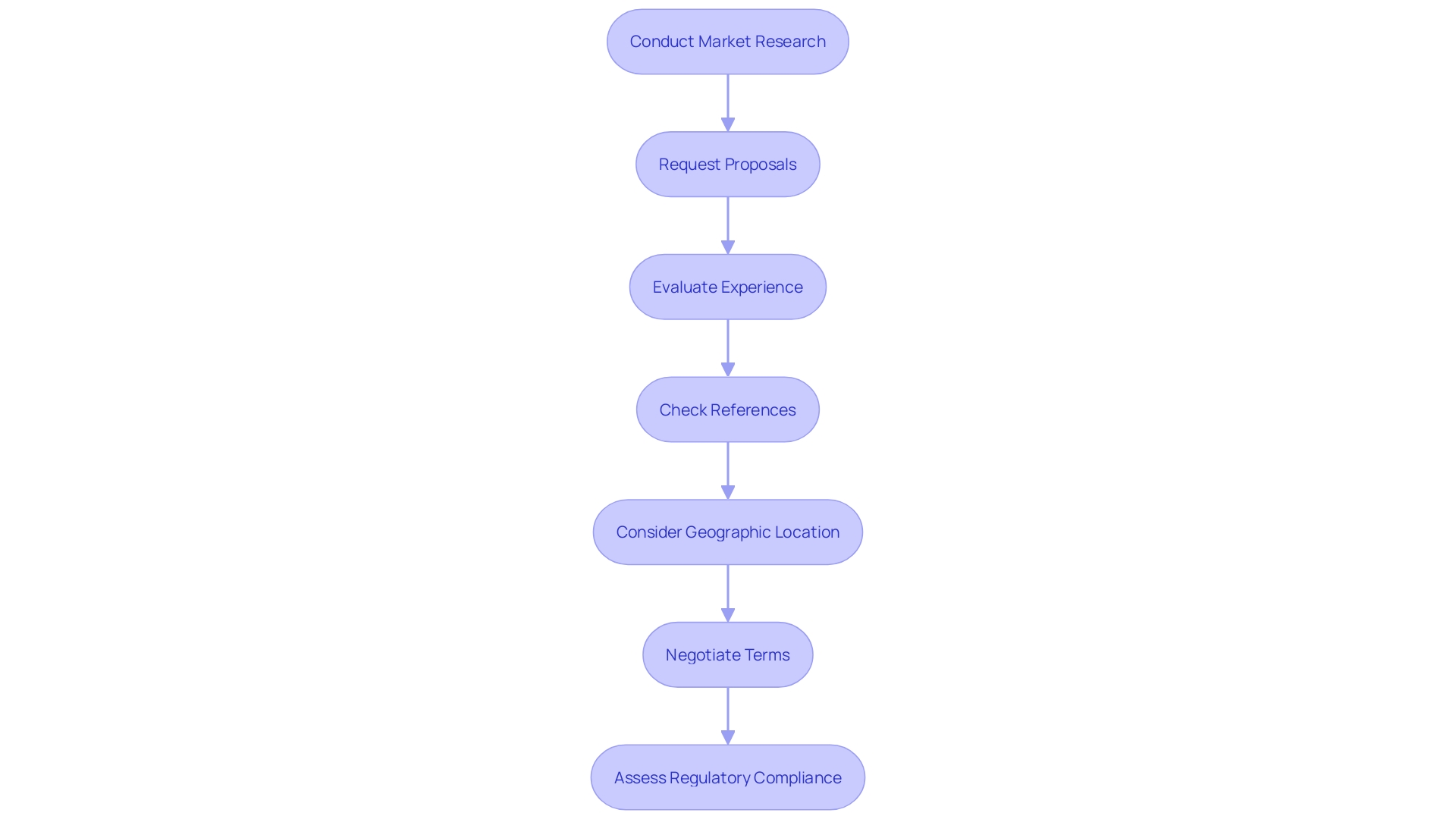
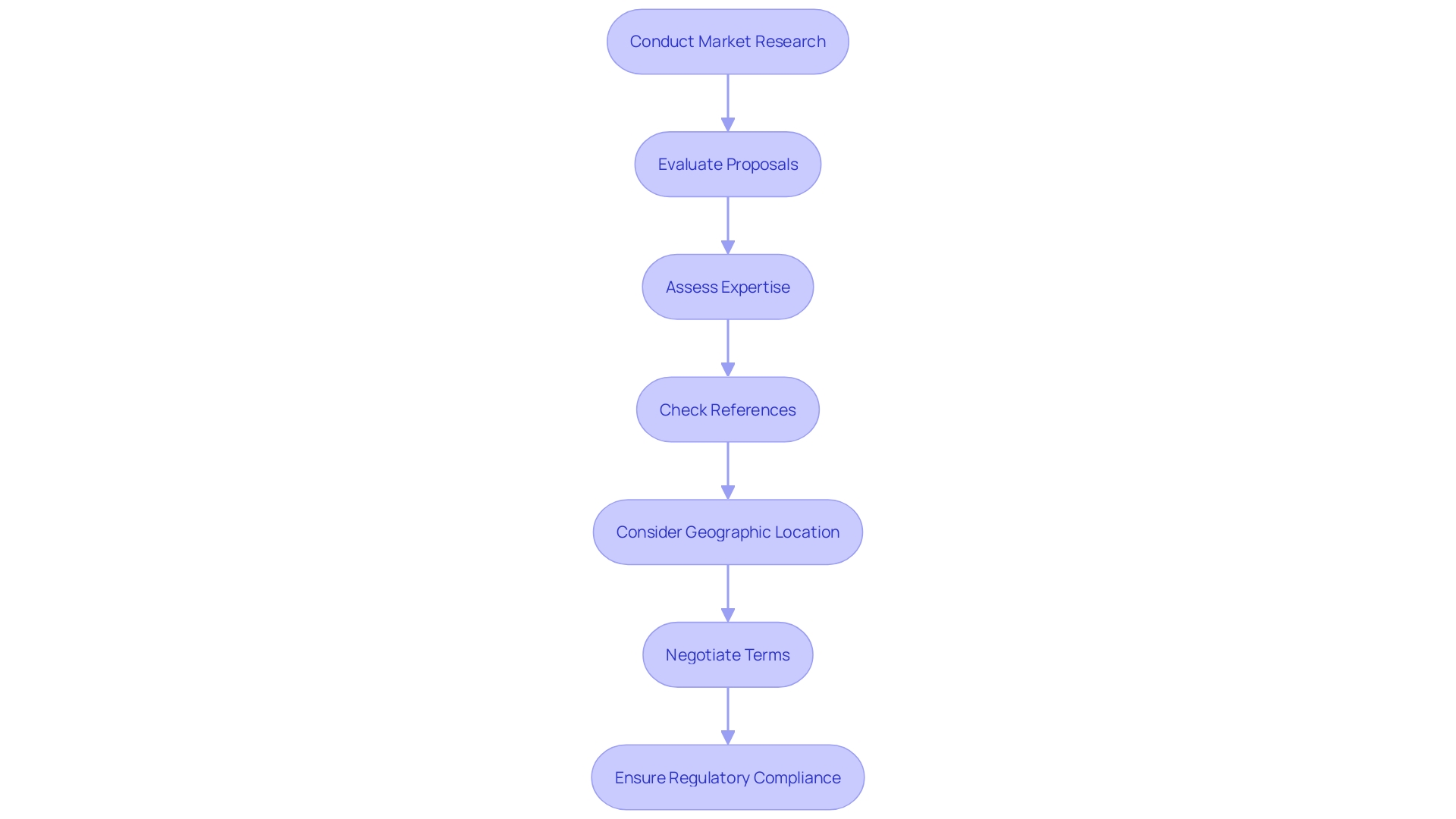
Conduct Thorough Market Research: Begin by investigating various CROs specializing in medical devices, such as bioaccess®, which provides accelerated clinical study services in Latin America. Focus on those with a proven track record in your specific research area, comparing their services, expertise, and pricing models to identify candidates that align with your budgetary constraints.
-
Request Detailed Proposals: Once you've narrowed down your options, request comprehensive proposals from the selected CROs. These proposals should encompass a breakdown of expenses and services provided, ensuring you pay attention to any hidden charges that may not be instantly clear.
-
Evaluate Experience and Expertise: Assess the CRO's experience in managing studies similar to yours. With over 20 years of expertise, bioaccess® excels in conducting Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up studies. This experience can help streamline processes and lower expenses through enhanced efficiency, even if their initial quote appears higher.
-
Check References and Past Performance: Reach out to previous clients to gauge their experiences with the CRO. Inquire about aspects such as timelines, budget adherence, and overall satisfaction. This feedback is crucial in determining whether the CRO can fulfill its commitments.
-
Consider Geographic Location: The location of the CRO can significantly impact costs. Choose CROs that are nearer to your study locations, as this may assist in reducing travel costs and logistical hurdles.
-
Negotiate Terms: Do not hesitate to negotiate terms. Discuss your budget constraints openly and explore whether the CRO is willing to offer flexible payment options or discounts for bundled services.
-
Assess Regulatory Compliance: Ensure that the CRO complies with all relevant regulatory guidelines for medical device research. Katherine Ruiz, a Regulatory Affairs expert at bioaccess®, can provide valuable insights into compliance issues that could lead to costly delays. Collaborating with a CRO that emphasizes regulations and assurance is crucial for a successful trial.
Assessing the Quality of Services Offered by the CRO
When evaluating a CRO, assessing the standard of services is as important as considering their cost. Here are key factors to focus on:
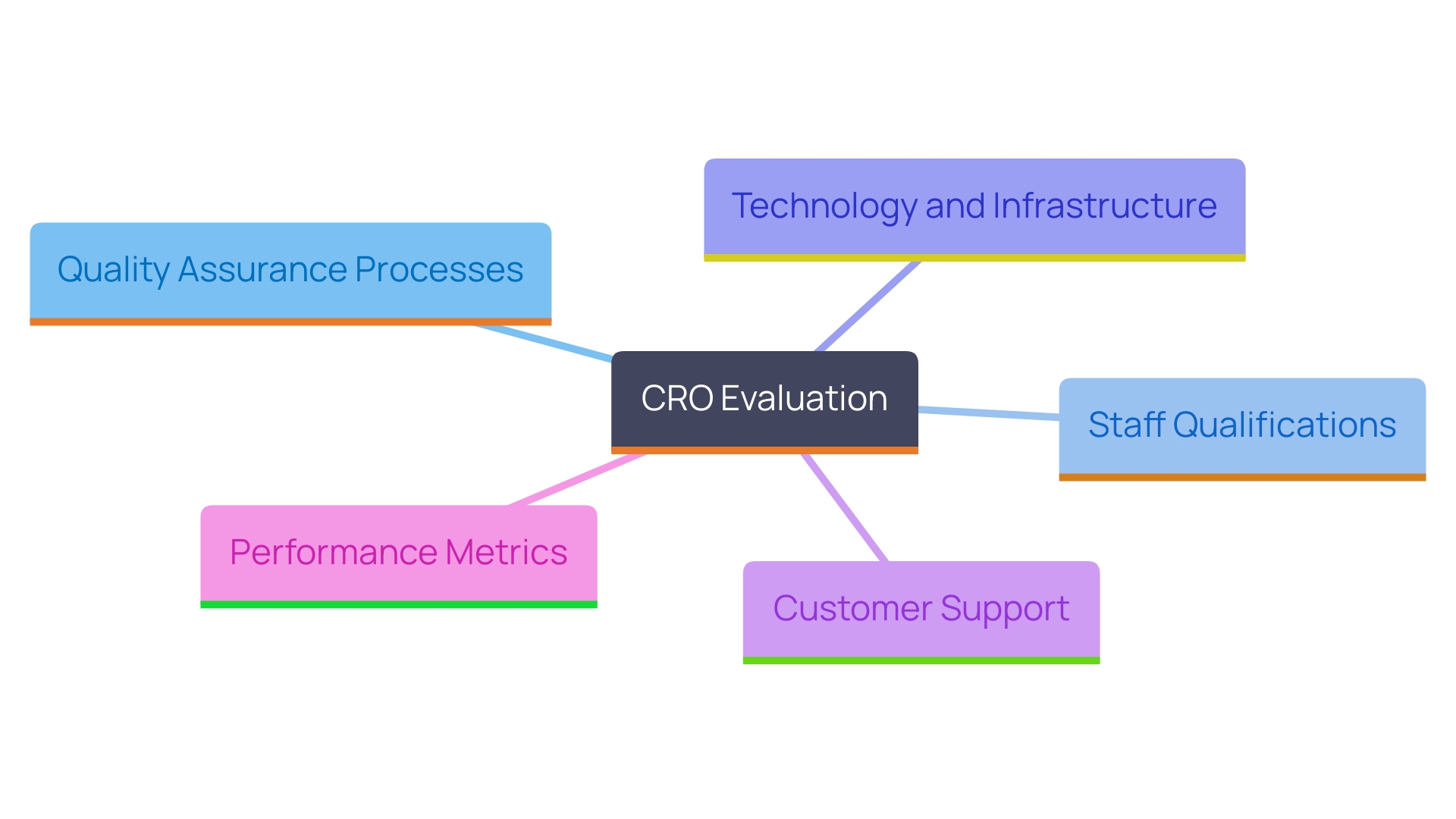
- Quality Assurance Processes: Inquire about the CRO's quality assurance measures. Do they have robust protocols in place to ensure compliance with regulatory standards, particularly in line with INVIMA's regulations for medical devices?
- Staff Qualifications: Review the qualifications and experience of the team that will be managing your project. Highly skilled researchers and managers can improve the standard of the study, especially in specialized areas like Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies.
- Technology and Infrastructure: Evaluate the technology and infrastructure utilized by the CRO. Advanced tools and systems can improve data collection, analysis, and reporting, contributing to high-quality outcomes in clinical trials.
- Customer Support: Assess the level of customer support provided. A CRO that offers responsive communication and assistance can substantially impact the overall experience and success of your research.
- Performance Metrics: Review performance metrics and client satisfaction ratings. These indicators can provide valuable insights into the CRO's ability to deliver excellent results consistently.
For instance, bioaccess® has demonstrated its commitment to excellence through successful collaborations in Colombia, leading to significant improvements in local healthcare outcomes and job creation. By focusing on these factors, you can ensure that your choice of CRO will not only meet regulatory compliance but also contribute positively to the local economy through enhanced healthcare services and international collaboration.
Making the Final Decision: Balancing Cost and Quality
After assessing potential CROs based on price, standards, and overall suitability, it’s essential to make a well-informed final choice. Here are steps to help you effectively balance cost and quality in your selection process:
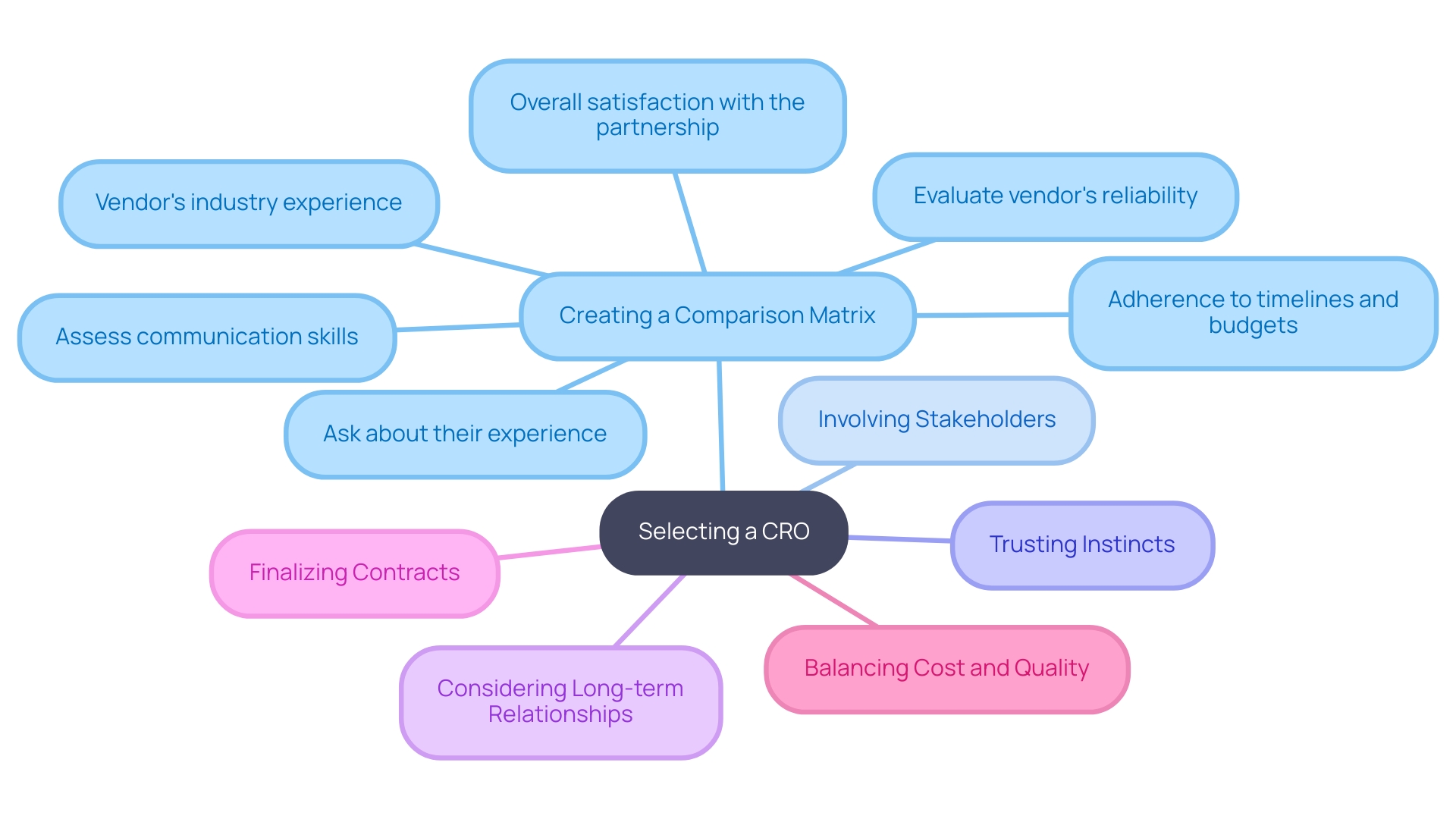
- Create a Comparison Matrix: Develop a matrix to evaluate each CRO against key criteria such as cost, experience, quality assurance, and client feedback. This visual tool will help you compare and contrast your options conveniently.
- Involve Key Stakeholders: Engage team members and stakeholders in the decision-making process. Their insights can offer valuable perspectives and ensure that all relevant factors are considered.
- Trust Your Instincts: While data and metrics are essential, don't underestimate the importance of intuition. If a particular CRO feels like the right fit based on your interactions and their understanding of your needs, trust your judgment.
- Consider Long-term Relationships: Think about the long-term implications rather than just short-term savings. A CRO like bioaccess™, with its extensive capabilities in feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting, may appear more expensive initially but can lead to superior quality and support, resulting in better outcomes and lower expenses over time.
- Finalize the Contract: Once you’ve made your choice, carefully review the contract terms. Ensure that all agreed-upon services, timelines, and costs are clearly outlined to avoid potential misunderstandings in the future. Partnering with a vetted organization like bioaccess™ can significantly enhance your clinical trial management process in Colombia.
Conclusion
Selecting the right Contract Research Organization (CRO) is a pivotal decision in the clinical research landscape for medical devices, particularly for Medtech startups in Latin America. The article emphasizes the importance of cost-effectiveness while underscoring that the quality of services should not be compromised. By conducting thorough market research, evaluating expertise, and assessing the quality of services, stakeholders can identify CROs like bioaccess® that offer both financial advantages and high standards of compliance.
The strategies outlined, from requesting detailed proposals to negotiating terms, provide a structured approach to making informed decisions. Balancing cost and quality through a comparison matrix and involving key stakeholders ensures that the chosen CRO aligns with both budgetary constraints and research goals. Ultimately, the right CRO partnership can lead to not only successful clinical trials but also contribute significantly to advancing healthcare innovation.
In conclusion, the selection of a CRO is a critical investment that requires careful consideration of various factors. By prioritizing both cost-effectiveness and service quality, organizations can achieve optimal results, paving the way for future advancements in medical technology and improved healthcare outcomes. This thoughtful approach will not only enhance the efficiency of clinical trials but also foster long-term relationships that drive success in the competitive Medtech arena.




