Introduction
In the realm of clinical research, the selection of a Contract Research Organization (CRO) is a pivotal decision that can significantly influence the outcome of medical device trials. As the landscape of clinical investigations evolves, particularly in the context of regulatory compliance and patient representation, understanding the intricacies of choosing the right CRO becomes essential. This article delves into the critical factors that organizations must consider when evaluating potential CRO partners, ranging from assessing their expertise and regulatory adherence to the importance of transparent communication and support.
By meticulously navigating these considerations, researchers can enhance the likelihood of successful trials that not only meet regulatory standards but also contribute meaningfully to the advancement of medical technology.
Identify Your Research Needs
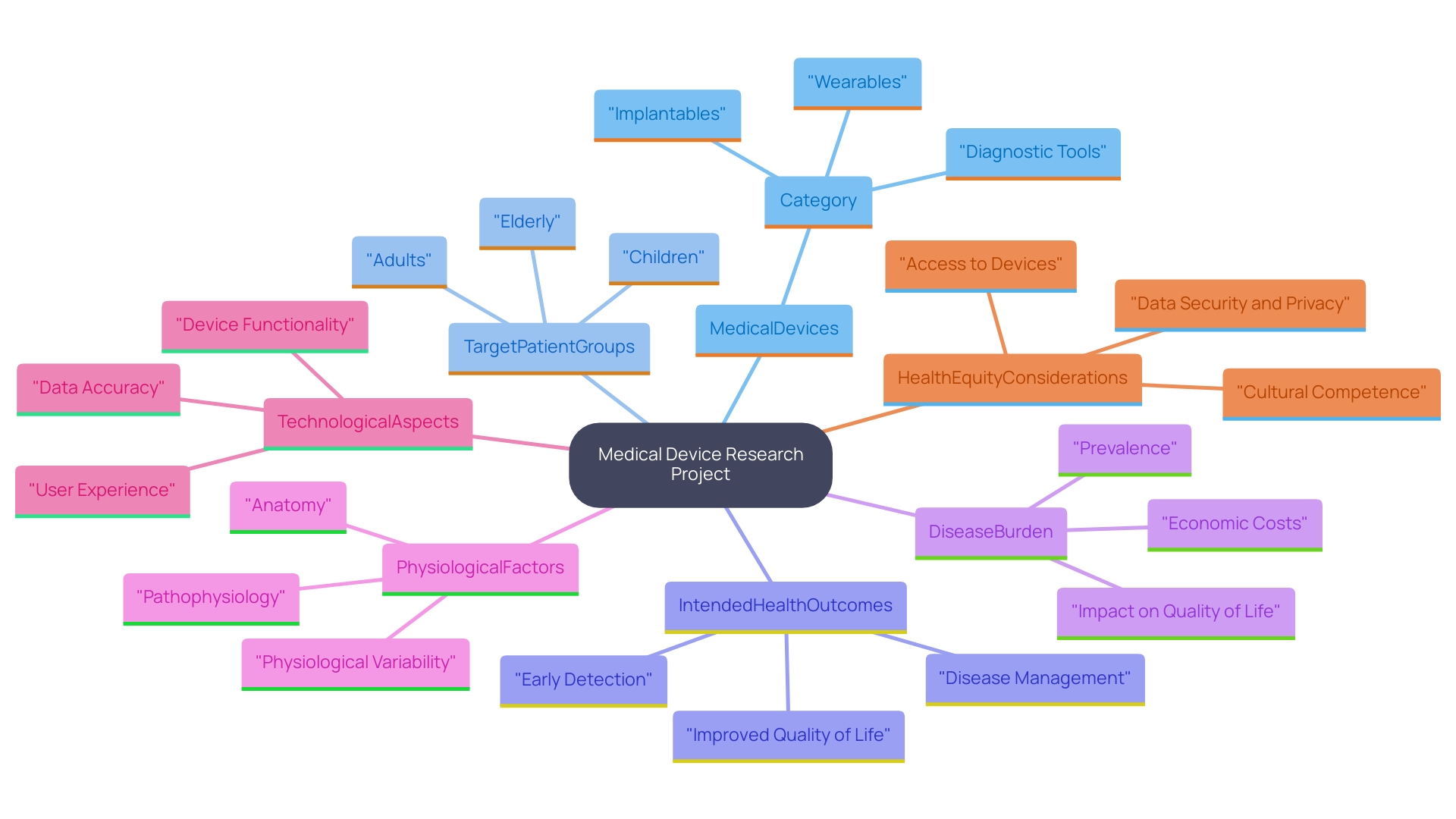
To ensure the success of your research project, it is essential to meticulously define its specific requirements. Start by determining the category of medical devices being examined, the target patient group, and the intended health outcomes. This foundational understanding will be instrumental in guiding your selection of a Contract Research Organization (CRO) that specializes in your area of interest.
In recent discussions surrounding research study design, several key considerations have emerged that are crucial for accurately reflecting the intended use population of medical devices. These considerations include the disease burden or condition being addressed, the physiological and anatomical factors pertinent to the patient population, and the technological aspects of the device itself. By integrating these elements into your project design, you can enhance the likelihood of generating relevant and generalizable data that truly reflects the diverse patient populations these devices serve.
Moreover, as the FDA emphasizes the importance of health equity in medical device research, it is increasingly vital to consider how your study population mirrors the broader community. The agency's initiatives aim to reduce barriers to health equity and improve health outcomes across various demographics, which can be achieved by carefully selecting study populations that represent the intended use of the device. These strategic decisions will not only facilitate compliance with regulatory guidelines but also ensure that the resulting data can effectively inform medical practice and enhance patient outcomes.
Evaluate CRO Experience and Expertise
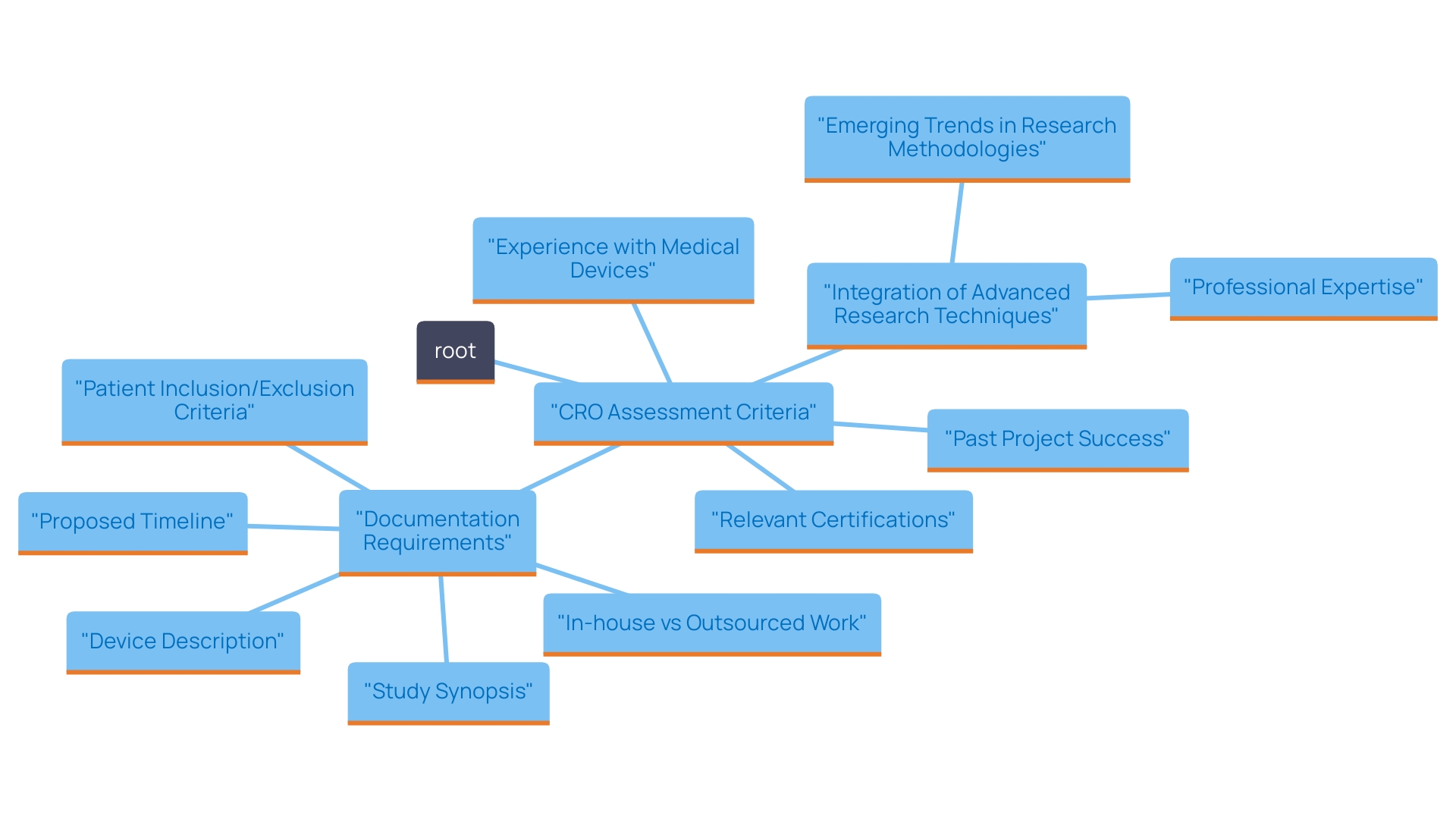
When choosing Contract Research Organizations in Paraguay, it is essential to carefully assess their experience and specialized knowledge in performing research studies, especially for medical devices. Focus on contract research organizations with a demonstrated history of managing successful trials, which can be evidenced by reviewing their past projects and publications. Furthermore, evaluate their pertinent certifications and accreditations, as these indicators of competence can significantly affect the quality of the research process.
For instance, CROs like bioaccess™ have successfully bridged the gap between innovative Medtech companies and the opportunities present in Latin America, offering cost-effective and high-quality services. Their ability to deliver reliable results is underscored by their team, which comprises professionals across various fields, including electrical, mechanical, and biomedical engineering, as well as medical and pharmaceutical sciences.
Furthermore, recent conversations at the Outsourcing in Clinical Trials: Medical Devices Europe 2024 conference underscored the changing environment of research studies, highlighting the significance of incorporating advanced techniques such as artificial intelligence and real-world data to improve regulatory submissions. The investigation of decentralized studies (DCTs) has also become a prominent topic, as these methods provide innovative solutions for participant engagement and data collection, further enhancing the efficiency and effectiveness of medical inquiries.
By examining the abilities and histories of possible CRO partners, you can guarantee that the chosen organization has the necessary knowledge and resources to carry out high-standard studies while managing the intricacies of regulatory adherence and project oversight.
Compare Pricing Structures
When selecting a contract research organization (CRO), it is crucial to request comprehensive pricing information from multiple providers. This entails a detailed comparison of their pricing structures, paying particular attention to any potential hidden costs, such as regulatory submission fees or data management expenses. Understanding the full scope of services included in their quoted prices is vital, as this will allow for a more accurate assessment of whether their offerings align with your budgetary constraints.
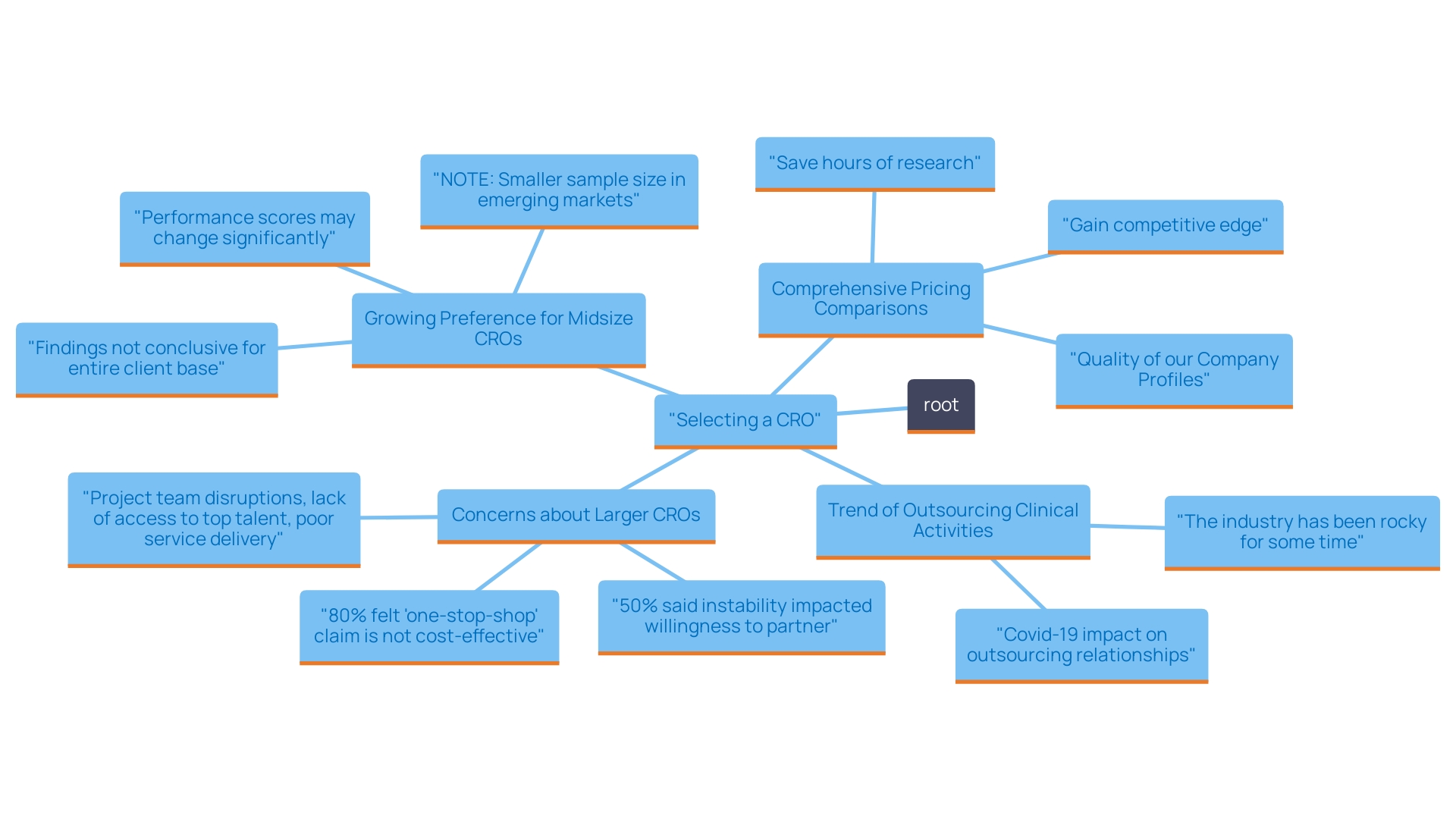
A significant trend in the industry indicates that nearly 70% of MedTech companies intend to outsource some of their clinical activities to contract research organizations or consultants this year, due to the considerable workload involved in planning, conducting, and analyzing clinical trials. This shift underscores the importance of finding a CRO that not only fits within financial parameters but also provides reliable and responsive service.
Surveys have indicated that 80% of respondents regard the 'one-stop-shop' claims of larger contract research organizations as less cost-effective, with half expressing concerns over the instability of these organizations, which can lead to project team disruptions and a lack of access to top talent. This sentiment highlights the growing preference for midsize contract research organizations, which are perceived to offer greater agility and personalized attention, with 83% of respondents citing these qualities as key factors in their decision-making process.
As you navigate this selection process, be sure to evaluate the transparency of communication and responsiveness of the contract research organizations you consider. The recent trend indicates a projected shift from large to midsize CROs, with a net gain of 5% expected over the next three years, suggesting that many in the industry are prioritizing a partnership that adapts to their evolving needs while ensuring confidence in service delivery.
Assess Regulatory Compliance and Quality Assurance
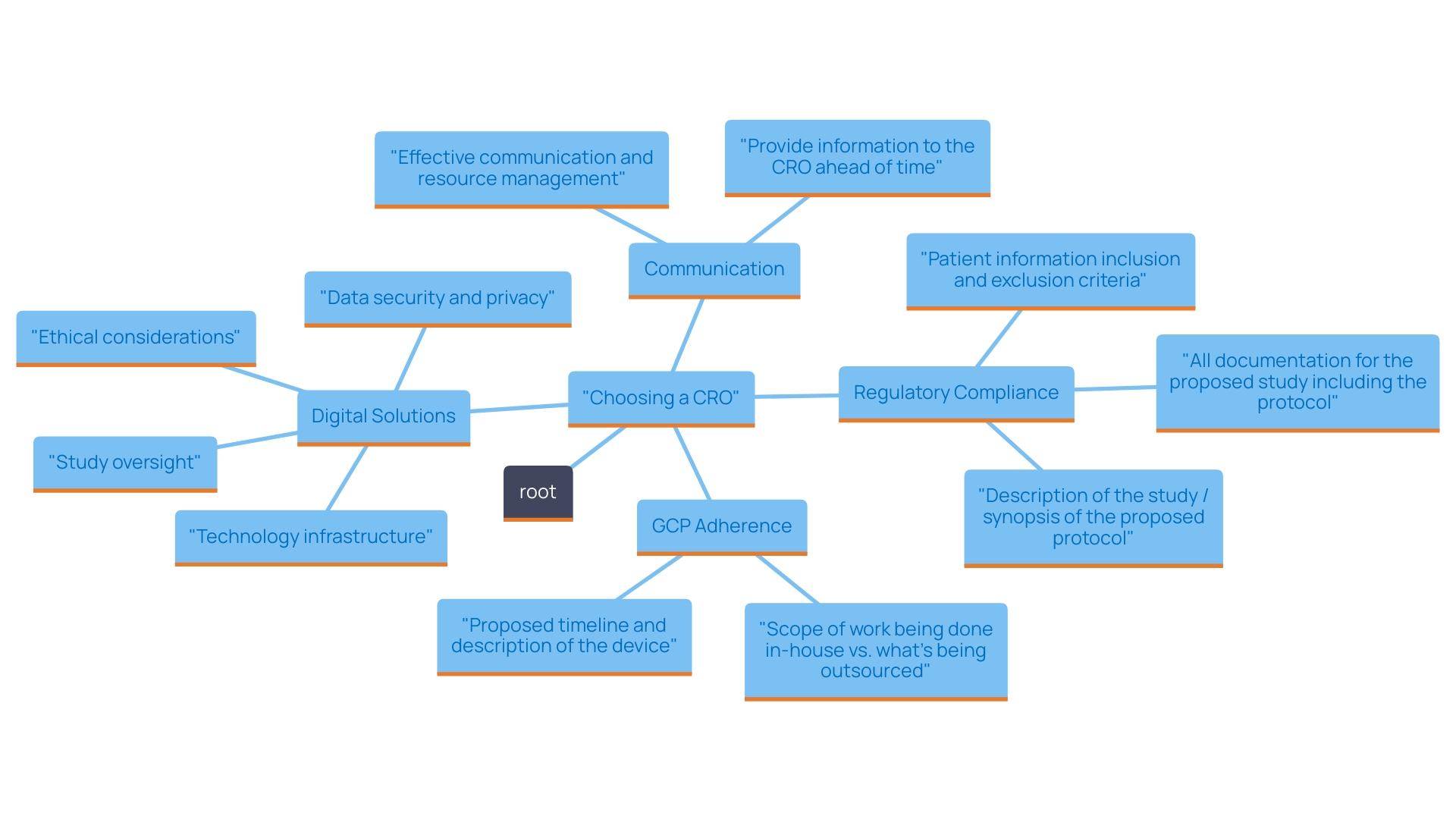
Choosing a Contract Research Organization (CRO) that complies with both local and international regulatory standards is essential for the success of your research. It is essential to inquire about their quality assurance processes and their strategies for ensuring compliance with Good Clinical Practice (GCP) guidelines. These standards not only safeguard the integrity of your study but also prioritize the safety of participants. With recent advancements in the regulatory landscape, such as the FDA's efforts to harmonize human subject protection regulations, a CRO's adherence to these guidelines can significantly streamline your research procedures. Furthermore, as the sector shifts towards more decentralized studies (DCT), grasping how a CRO utilizes digital solutions for oversight and administration grows more vital. By utilizing innovative planning tools that monitor waste reduction and improve communication among research teams, organizations can lessen resource wastage caused by unexpected alterations in study plans. Consequently, this proactive approach not only fosters regulatory compliance but also enhances patient trust and engagement, ultimately leading to more successful trial outcomes.
Evaluate Communication and Support
Evaluating the communication practices of a Contract Research Organization (CRO) and the level of support they provide throughout the research process is essential for effective project management. Strong communication serves as the backbone of successful collaboration, ensuring that all stakeholders are kept informed and engaged from the initial phases through to project completion. It is critical to verify that the CRO assigns a dedicated project manager who acts as the primary liaison between your team and the CRO, facilitating timely updates and addressing any concerns that may arise.
Moreover, a responsive support system is crucial in navigating the complexities of clinical research. This system should not only be accessible but also proactive in identifying potential issues and implementing solutions. For instance, organizations like De Montfort University have demonstrated the importance of well-defined workflows and support materials to cultivate an active research community. Dr. Aamir Hussain, the Research Data Project Officer, exemplified this by fostering engagement through strategic communication and resource management, despite limited resources.
According to a global survey in The State of Open Data 2023, nearly 75% of researchers reported never receiving support for data sharing, underlining the necessity for contract research organizations to develop robust support frameworks. In light of increasing expectations from funders for open data practices, clinical research organizations must prioritize efficient communication and support mechanisms to enhance researcher engagement and compliance. By establishing clear channels for message dissemination and ensuring that researchers feel supported throughout the process, CROss can significantly reduce the potential for misunderstandings and project delays.
Seek References and Testimonials
When selecting a contract research organization (CRO), it is crucial to gather references from previous clients and seek testimonials that reflect the CRO's performance. This feedback can illuminate various aspects of the CRO's operations, including their reliability, quality of work, and overall client satisfaction. Engaging with former clients can provide rich insights, highlighting both strengths and potential areas for improvement. Additionally, tapping into your professional network can be highly beneficial; reaching out to industry colleagues for recommendations can uncover valuable perspectives on a CRO's capabilities. Such collaborative insights can significantly enhance the decision-making process, allowing for a more informed choice that aligns with the specific requirements of your research projects. Given the complexities of the MedTech landscape, where nearly 70% of companies are planning to outsource clinical activities this year, ensuring that you choose a highly regarded and capable CRO is paramount for success.
Conclusion
The selection of a Contract Research Organization (CRO) is a critical step in ensuring the success of clinical trials for medical devices. By identifying specific research needs and understanding the target patient population, organizations can align their objectives with the right CRO that possesses the necessary expertise and regulatory knowledge. Evaluating the experience and track record of potential CRO partners is essential, as this ensures that they can navigate the complexities of clinical trials while adhering to industry standards.
Moreover, careful consideration of pricing structures can prevent unexpected costs and ensure that the chosen CRO offers value without compromising on quality. As the landscape of clinical research evolves, prioritizing regulatory compliance and quality assurance becomes increasingly important. CROs that embrace modern methodologies and digital solutions can enhance trial efficiency and participant safety.
Effective communication and support throughout the research process are equally vital. Organizations should seek CROs that provide dedicated project managers and proactive support systems to facilitate collaboration and address challenges promptly. Gathering references and testimonials from previous clients can further inform decisions, providing insights into a CRO's reliability and performance.
In summary, the right CRO partnership can significantly influence the outcomes of clinical trials. By meticulously evaluating potential partners based on expertise, compliance, communication, and client feedback, organizations can enhance their prospects for successful trials that contribute to the advancement of medical technology and improve patient care.




