Introduction
Selecting the right Contract Research Organization (CRO) for first-in-human studies is a critical step that can significantly influence the success of clinical trials, particularly in Latin America. With the region's unique regulatory landscape and growing opportunities for medical device research, organizations must navigate a complex array of criteria when making their choice. This article delves into the essential factors to consider, from assessing a CRO's experience and regulatory compliance to understanding the importance of local expertise and communication strategies.
By exploring these key aspects, stakeholders can ensure they partner with a CRO that not only meets their specific needs but also enhances the overall efficiency and effectiveness of their clinical research endeavors.
Key Criteria for Selecting a CRO for First-in-Human Studies
When choosing a CRO for first-in-human research in Latin America, consider the following key criteria:
- Experience with First-in-Human Research: Ensure the CRO, such as bioaccess®, has a proven track record in conducting first-in-human investigations, as these activities require specialized knowledge and expertise.
- Regulatory Compliance: Verify that the CRO adheres to local and international regulatory standards, including Good Clinical Practice (GCP) guidelines, which are critical for successful trial outcomes.
- Therapeutic Expertise: Look for a CRO with experience in the specific therapeutic area relevant to your research, as this can significantly impact the success of the project.
- Quality Assurance Processes: Assess the CRO’s quality control and assurance measures to ensure they maintain high standards throughout the research process.
- Staff Qualifications: Evaluate the qualifications and experience of the CRO’s team members, particularly those who will be directly involved in your study, such as principal investigators.
- Project Management Capabilities: Consider the CRO's ability to manage timelines and budgets effectively, alongside their communication practices, essential for a smooth process.
- Site Selection and Management: Investigate how the CRO selects and manages clinical study sites to ensure they have access to appropriate patient populations and facilities, crucial for the success of your research.
- Technological Infrastructure: Ensure the CRO utilizes advanced technologies for data management and monitoring, which can enhance research efficiency and data integrity.
- Specific Services Offered: bioaccess® provides comprehensive services including feasibility and selection of research locations, setup and approval of assessments, import permits, project management, and detailed reporting on project status and adverse events.
- Vetted Provider Status: As the sole CRO approved as a business service provider for U.S. medical device firms in Colombia, bioaccess® provides distinct benefits in maneuvering through regulatory landscapes.
By carefully assessing these criteria, you can choose a First-in-Human Studies CRO in the Dominican Republic like bioaccess® that not only satisfies the requirements of your first-in-human trial but also enhances its overall success, utilizing their proficiency in extensive trial management services throughout Latin America.
Navigating the Clinical Research Landscape in the Dominican Republic
Conducting clinical research in the Dominican Republic, especially for first-in-human trials, is often managed by a First-in-Human Studies CRO Dominican Republic, which navigates a unique regulatory and operational landscape. Here are key points to consider:
-
Regulatory Framework: Familiarize yourself with the regulations set forth by the Dominican Republic's National Health Authority (SENASA) and the Ministry of Public Health.
Grasping these regulations is essential for adherence during the research, particularly when handling the intricacies of medical device trials. -
Ethics Committees: Engage with local ethics committees early in the planning process. Their approval is necessary for research initiation and can influence the timeline of your investigation.
bioaccess® can assist in navigating this process efficiently. -
Cultural Considerations: Recognize the cultural context in which you will be conducting research. Engaging with local communities and understanding their perspectives can enhance recruitment and retention, ensuring that studies resonate well within the community.
-
Site Availability: Evaluate the availability of trial locations and their capabilities. The Dominican Republic has an increasing number of research locations that may provide access to varied patient groups, essential for successful results in trials.
-
Local Expertise: Collaborate with local experts who understand the nuances of conducting research in the region. Their insights can be invaluable for navigating challenges and enhancing your research's compliance and execution.
-
Logistical Considerations: Plan for logistical aspects such as patient recruitment, transportation, and data management, which may differ from those in other countries. A thorough logistical plan is crucial for the smooth operation of research studies.
-
Partnerships: Consider establishing collaborations with local institutions or universities that can provide additional support and resources for your research. Partnerships such as those between bioaccess® and Greenlight Guru can enable quicker and more efficient trials.
By comprehending and maneuvering through the research environment in the Dominican Republic, you can improve the chances of a successful first-in-human trial in collaboration with a First-in-Human Studies CRO Dominican Republic while ensuring adherence to local regulations. This approach not only contributes to regulatory success but also transforms lives through access to advanced Medtech innovations. Furthermore, bioaccess® focuses on extensive management services for research, encompassing feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting, especially for Early-Feasibility, as well as First-in-Human Studies CRO Dominican Republic, Pilot, Pivotal, and Post-Market Follow-Up Research.
Evaluating CRO Capabilities and Services
When evaluating CRO capabilities and services, consider the following:
-
Study Design Support: Evaluate if the CRO can aid in creating the research protocol and offer insights on methodologies that adhere to regulatory standards, as demonstrated in the partnership between bioaccess™ and Caribbean Health Group to improve trials in Barranquilla, backed by Colombia's Minister of Health.
-
Patient Recruitment Strategies: Inquire about the CRO’s strategies for patient recruitment and retention. Notably, GlobalCare Clinical Trials (GCCT) has achieved over a 50% reduction in recruitment time and maintained a retention rate exceeding 95%, highlighting effective patient engagement strategies critical for the success of the First-in-Human Studies CRO Dominican Republic.
-
Data Management and Analysis: Evaluate the CRO's data management systems and their capabilities for statistical analysis to ensure robust data integrity and reporting.
-
Monitoring and Reporting: Understand the CRO’s approach to monitoring research progress and reporting findings, as this impacts the transparency and accountability of the research.
-
Regulatory Submission Support: Confirm whether the First-in-Human Studies CRO Dominican Republic offers assistance with regulatory submissions and interactions with health authorities, which can streamline the approval process and support compliance, particularly in the context of early-feasibility and first-in-human studies.
-
Budget Management: Discuss how the CRO manages budgets and financial reporting to avoid unexpected costs during the study.
-
Comprehensive Clinical Trial Management Services: bioaccess® offers a variety of specialized services for accelerated medical device trials, including Early-Feasibility Assessments (EFA), First-In-Human Trials (FIH), Pilot Trials, Pivotal Trials, and Post-Market Clinical Follow-Up Assessments (PMCF).
Their knowledge guarantees that every research project is handled efficiently, from feasibility evaluations to regulatory adherence and project oversight.
By thoroughly assessing these factors, you can confirm that the First-in-Human Studies CRO Dominican Republic you choose is adequately prepared to manage the intricacies of your first-in-human research, utilizing their extensive management services.
Establishing Clear Communication Channels
To establish clear communication channels with your chosen CRO, especially when navigating the complexities of first-in-human and early-feasibility research in Latin America:
- Define Roles and Responsibilities: Clearly outline the roles and responsibilities of each team member from both your organization and the CRO to avoid confusion.
- Routine Gatherings: Arrange routine gatherings to review research advancements, tackle issues, and implement essential modifications to the research framework, utilizing the 20+ years of experience from bioaccess® in overseeing health evaluations, including Pilot Studies and Post-Market Health Follow-Up Studies.
- Use Collaborative Tools: Utilize project management and communication tools (such as Slack, Trello, or Microsoft Teams) to facilitate real-time updates and information sharing.
- Establish Reporting Protocols: Set up protocols for reporting adverse events, data discrepancies, and other critical issues to ensure timely resolution, a practice supported by bioaccess®’s comprehensive reporting services that include status updates and inventory management.
- Feedback Mechanisms: Establish feedback systems to consistently enhance communication practices and tackle any issues that emerge during the research, in line with bioaccess®’s dedication to tailored project management and compliance evaluations.
By emphasizing communication and utilizing bioaccess®'s proficiency in clinical research management, including their skills in setup and project oversight, you can cultivate a cooperative atmosphere that improves the efficiency and effectiveness of your projects at the First-in-Human Studies CRO Dominican Republic.
Finalizing the Contract and Agreement Terms
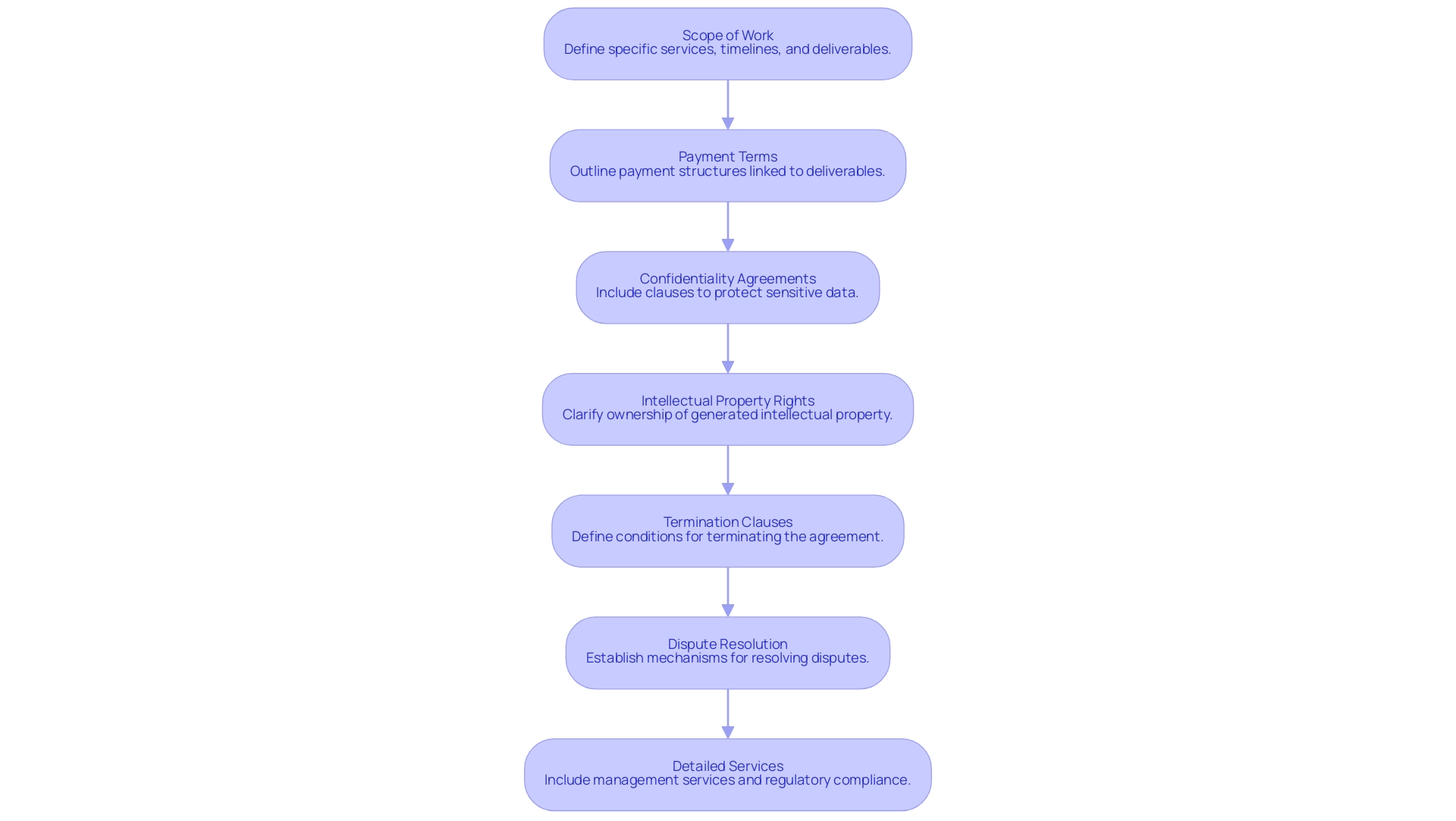
When finalizing the contract with your chosen CRO, ensure the following elements are included:
-
Scope of Work: Clearly define the scope of work, including specific services to be provided, such as feasibility assessments, site selection, and trial setup, along with timelines and deliverables.
-
Payment Terms: Outline payment structures, including milestones for payments linked to specific deliverables or stages of the project.
-
Confidentiality Agreements: Include confidentiality clauses to protect sensitive data and proprietary information from both parties.
-
Intellectual Property Rights: Clarify the ownership of intellectual property generated during the research to avoid disputes later.
-
Termination Clauses: Define termination clauses that outline the conditions under which either party can terminate the agreement.
-
Dispute Resolution: Establish mechanisms for resolving disputes that may arise during the collaboration to ensure a fair process.
-
Detailed Services: Ensure the CRO's responsibilities include comprehensive management services such as regulatory compliance, project management, and reporting on study status and adverse events. Additionally, include specific services related to the submission processes for the ethics committee and the Ministry of Health (INVIMA), as well as the import permit application to Colombia's Ministry of Industry, Commerce, and Tourism (MinCIT).
These elements are crucial for navigating the complex regulatory landscape in Colombia, where the approval process can be lengthy and challenging.
By carefully reviewing and finalizing these contract terms, you can establish a solid foundation for a successful partnership with your CRO, effectively addressing the unique challenges faced by medical device startups in clinical trials.
Conclusion
Selecting the appropriate Contract Research Organization (CRO) for first-in-human studies in Latin America is a multifaceted decision that can greatly affect the trajectory of clinical trials. The key criteria for evaluation, such as the CRO's experience, regulatory compliance, and therapeutic expertise, are paramount in ensuring a successful partnership. Additionally, understanding the local regulatory framework and cultural context is vital for navigating the complexities of conducting research in regions like the Dominican Republic.
Establishing clear communication channels and defining roles within the partnership further enhances collaboration, which is essential for the smooth execution of clinical trials. Moreover, finalizing contract terms with attention to detail, including scope of work and payment structures, lays a solid foundation for the relationship between the sponsor and the CRO.
Ultimately, a thorough evaluation of these factors not only aids in selecting a CRO that aligns with specific study needs but also contributes to the overall success of first-in-human studies. By leveraging the expertise of a capable CRO, stakeholders can navigate the intricate landscape of clinical research more effectively, ensuring compliance and fostering innovation in medical technology.




