Introduction
In the realm of medical device trials, the selection of a Contract Research Organization (CRO) is a pivotal decision that can significantly influence the success of clinical research. As the landscape of medical technology evolves, understanding the intricacies involved in choosing the right CRO becomes essential for stakeholders aiming to navigate regulatory complexities and ensure patient-centric outcomes. This article delves into the critical steps necessary for effectively evaluating and selecting a CRO, from defining research needs and assessing qualifications to comparing proposals and finalizing agreements.
By adopting a systematic approach, organizations can enhance their chances of conducting successful trials, ultimately contributing to advancements in healthcare innovation.
Understand Your Research Needs
Establishing the objectives and requirements for your medical apparatus study is an essential initial action that lays the groundwork for successful research. Begin by identifying the specific expertise necessary for your study, which may include considerations related to the type of medical product being tested, the target patient population, and the study design. Recent trends indicate a growing emphasis on patient-centric approaches, prioritizing the experiences and outcomes that matter most to patients, thereby shaping how objectives are framed.
This fundamental comprehension not only guides the choice of suitable Contract Research Organizations (CROs) but also corresponds with changing trends in medical product testing requirements, such as heightened emphasis on regulatory compliance and post-market monitoring. Essential steps encompass feasibility studies, site selection, compliance reviews, experiment setup, import permits, project management, and comprehensive reporting of study status and adverse events.
However, medical device startups often face significant challenges, including financial constraints that limit their ability to conduct thorough evaluations and difficulties in patient recruitment due to reluctance or ineligibility. As noted by various leaders in the field, including Albert Einstein, research is fundamentally about navigating the unknown: 'If we knew what we were doing, it would not be called research.' This viewpoint highlights the significance of distinctly outlining research requirements, improving the accuracy and efficacy of clinical studies.
When setting your objectives, consider including specific endpoints related to safety and efficacy, as well as regulatory compliance. Establishing clear metrics for patient outcomes not only aligns with recent regulatory expectations but also fosters greater transparency and trust in the research process. By articulating these objectives, you create a robust framework that guides decision-making and ensures that your study meets both scientific and regulatory standards. Moreover, leveraging the expertise of CROss can help mitigate regulatory hurdles, making the process smoother for startups navigating these complexities.
Research Potential CROs
When choosing Contract Research Organizations (CROs) for medical equipment studies in Panama, it is essential to take into account those with a solid reputation, pertinent experience, and favorable reviews from former clients. Research indicates that choosing the right CRO can significantly influence the success of medical device development. Significantly, partnerships such as that of bioaccess™ and Caribbean Health Group seek to establish Barranquilla as a premier location for medical research in Latin America, backed by Colombia's Minister of Health. Additionally, GlobalCare Clinical Studies has collaborated with bioaccess™ to improve ambulatory services for studies in Colombia, achieving over a 50% decrease in recruitment time and maintaining a retention rate of over 95%. Here is a curated list of notable CROs specializing in medical device trials within the region:
-
SGR Clinical Research: Recognized for its expertise in clinical trial design, SGR has established a strong reputation, particularly in the field of chronic wound management. Dr. Thomas Serena states, 'SGR has set the standard in clinical study design for chronic wounds, such as diabetic foot ulcers.' Their creative methods have resulted in numerous successful experiments and product approvals.
-
Greenlight Guru Clinical: Established by Páll Jóhannesson, this organization concentrates on enhancing the regulatory process for medical technologies, ensuring compliance and efficiency throughout trials. Their commitment to excellence has made them a trusted partner for many companies in the industry.
-
Axonal-Biostatem: Located in Nanterre, France, this CRO offers specialized services related to medical instruments. Although specific outcomes from their work are not detailed, it is known that they have successfully contributed to multiple product approvals in the European market, indicating a commitment to enhancing medical research.
In selecting a CRO, companies should evaluate expertise in relevant therapeutic areas, a proven track record, and global reach. Utilizing online resources, industry publications, and professional networks can help gather testimonials and insights into the effectiveness and reputation of these organizations. For instance, Dr. John B. Simpson, CEO of Avinger, has shared positive experiences regarding OCT-guided atherectomy research conducted at a site in Cali, Colombia, stating, 'Working with bioaccess™ has streamlined our research process and improved our outcomes significantly.' By prioritizing these factors, clinical researchers in Panama can identify reputable CROss that align with their project needs and objectives, ultimately enhancing the likelihood of successful research outcomes.
Evaluate CRO Qualifications
When evaluating the qualifications of Contract Research Organizations (CROs) on your shortlist, it is crucial to adopt a systematic approach. Start by examining their history in executing similar medical equipment studies, particularly their familiarity with the regulatory environment in Panama, which is crucial for guaranteeing adherence and favorable study results.
Accreditation and certifications are key indicators of a CRO's credibility and capability. Examine if the organization possesses pertinent accreditations, especially those that correspond with international standards for medical equipment studies, such as ISO 13485 and Good Clinical Practice (GCP). Such credentials not only demonstrate adherence to quality practices but also reflect their commitment to maintaining high operational standards.
Furthermore, the expertise of the CRO's team members plays a significant role in the quality of services provided. For example, experts such as Juan Cuya, MD, provide significant experience in regulatory matters and management of research studies, while Katherine Ruiz contributes specialized insights in Regulatory Affairs for medical equipment and diagnostic tests in Colombia. Evaluate their qualifications, experience, and previous participation in medical research to determine their proficiency in navigating complex regulatory environments. As remarked by industry specialist Dr. Jane Smith, "Regulatory experience is not merely a necessity; it is a fundamental element of successful research, particularly in the changing environment of medical instruments."
Alongside these elements, take into account the expedited medical equipment research services available in Latin America, like those provided by bioaccess®, which focus on early-feasibility, first-in-human, pilot, pivotal, and post-market follow-up studies. Considering the increasing need for CRO services, fueled by a rise in outsourcing for pharmaceutical research and development, verifying that your chosen CRO has these qualifications will improve efficiency and productivity in your studies. With the market projected to expand at a CAGR of 9.3% from 2024 to 2032, selecting the right CRO can be a strategic advantage in this evolving landscape.
Consider Regulatory Compliance and Quality Assurance
Choosing a Contract Research Organization (CRO) that shows a strong system for adhering to regulatory requirements specific to medical device studies is essential for the success of your research. It is essential to inquire about their quality assurance processes, particularly regarding how they maintain data integrity and prioritize patient safety. A CRO that is deeply committed to regulatory compliance can significantly mitigate risks throughout your study.
For instance, a case study involving XYZ CRO illustrates how they implemented a comprehensive quality assurance system that led to a 30% reduction in regulatory compliance issues over two years. This improvement was achieved through regular training sessions and audits that emphasized the importance of adhering to regulatory standards.
The principles described in the Deming Cycle—plan-do-check-act—act as an essential tool for ongoing enhancement in quality management of research studies. Implementing this model ensures that quality issues are addressed systematically, enhancing compliance and operational efficiency.
Moreover, effective corrective and preventive actions (CAPA) necessitate thorough root cause analysis. For example, a recent implementation of CAPA at ABC Medical Devices involved identifying the root cause of data discrepancies through detailed investigations, which subsequently led to the development of a more robust data verification process. This method emphasizes creating sustainable solutions to recognized issues, thereby strengthening the integrity of the research process.
As Arun Bhatt, President of Clininvent Research Pvt. Ltd., stated, 'The Clinical Trials Transformation Initiative (CTTI) has characterized quality as the ability to effectively answer the intended question about the benefits and risks of a medical product while assuring protection of human subjects.' By prioritizing these aspects, you can ensure that your chosen CRO not only meets regulatory standards but also upholds the highest quality assurance practices.
Moreover, utilizing the knowledge of experts such as Katherine Ruiz, who focuses on regulatory matters for medical products and in vitro diagnostics in Colombia, can offer invaluable insights into navigating intricate regulatory environments. Moreover, collaborating with prominent organizations like bioaccess®, recognized for their emphasis on innovation and regulatory excellence in Latin America, can improve your study's success and adherence.
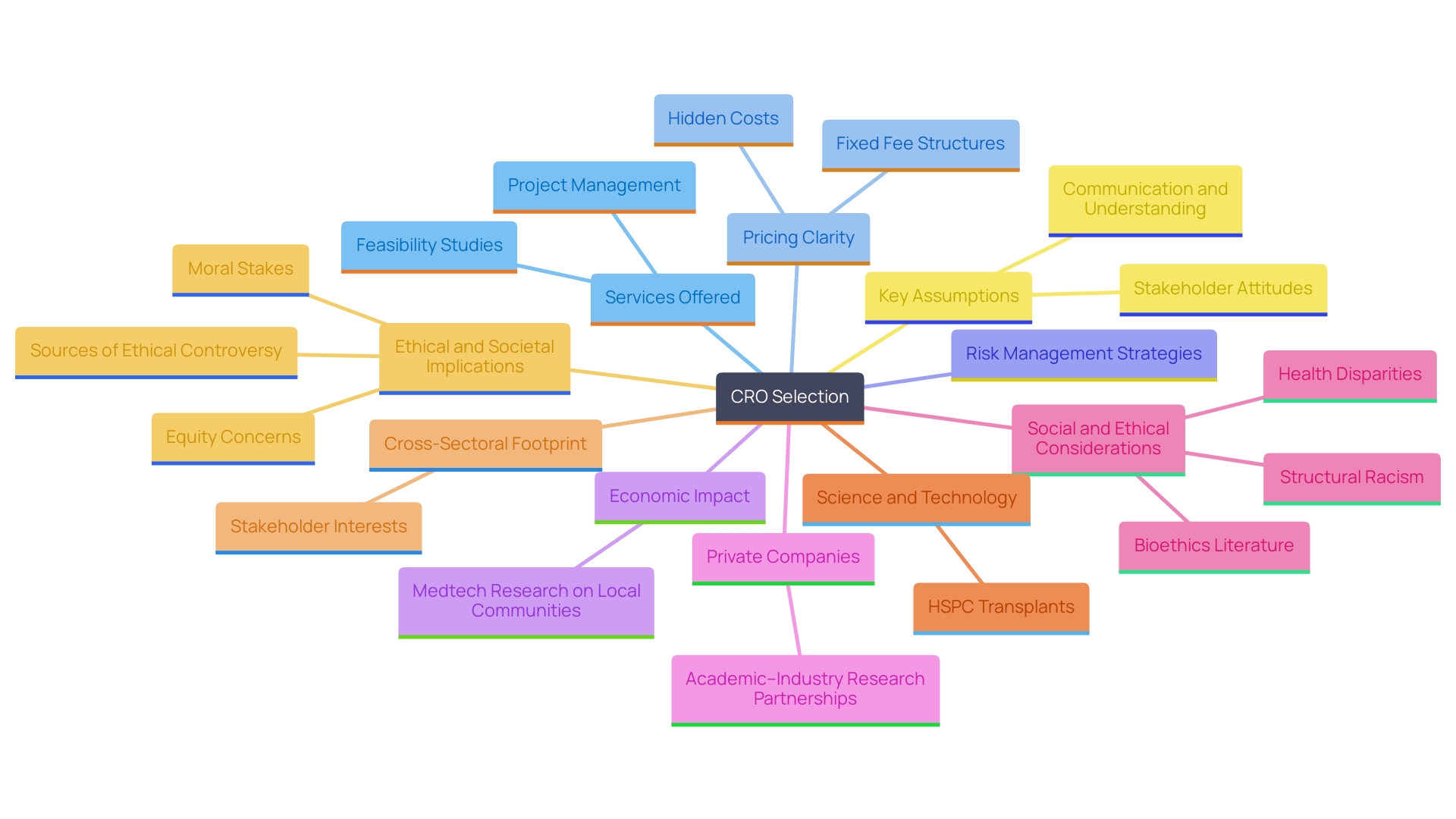
Request Proposals and Compare Costs
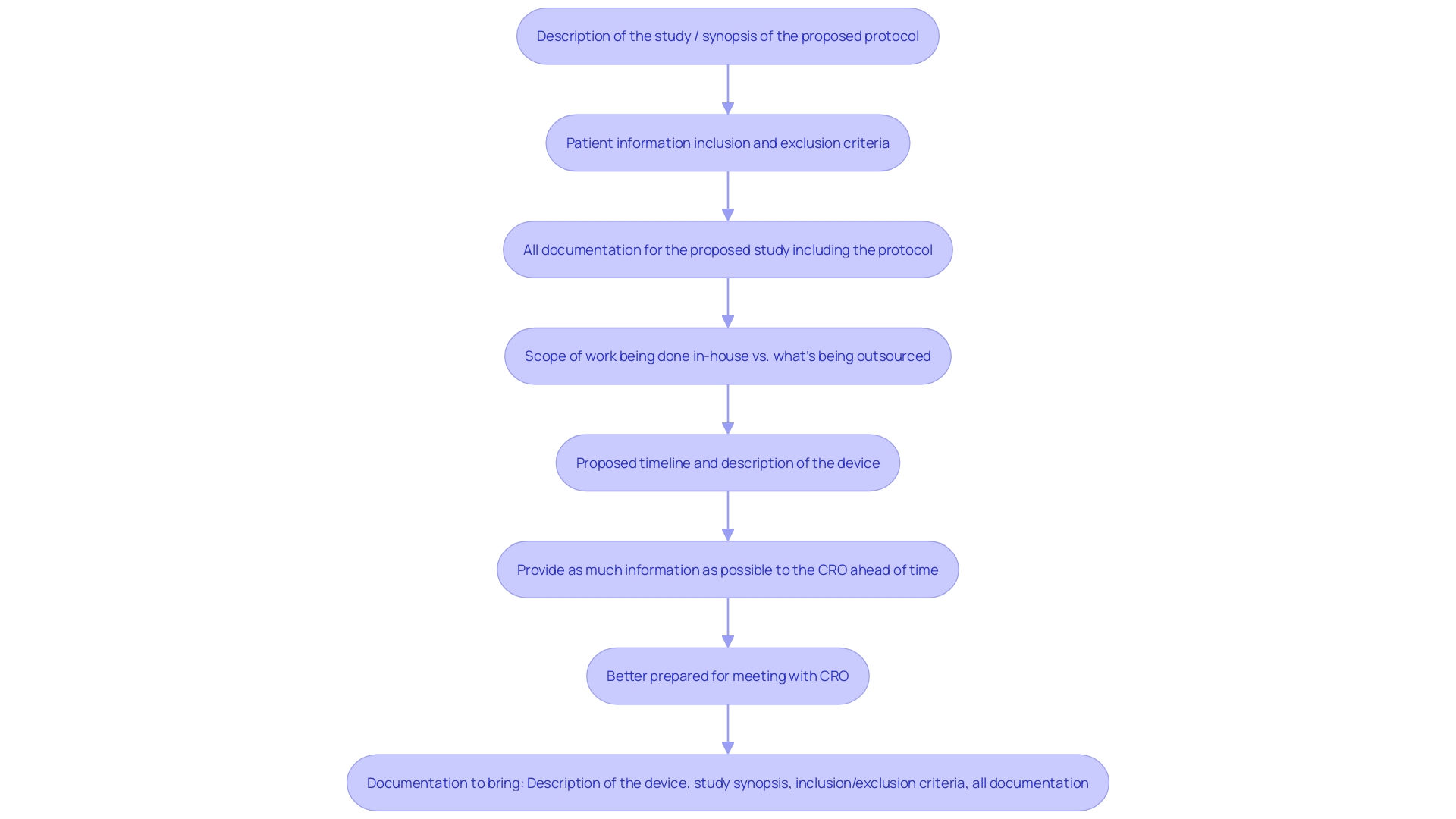
When assessing potential Contract Research Organizations (CROs) for medical device studies, it is essential to request detailed proposals that clearly outline their services, timelines, and costs. A thorough comparison of these proposals should extend beyond merely examining the bottom line; it should also emphasize the overall value each CRO offers, including their expertise in comprehensive clinical research management services such as feasibility studies, site selection, compliance reviews, setup, import permits, project management, and reporting.
Transparency in pricing is paramount, as hidden costs can significantly affect the budget. For instance, a protocol change that extends a study by four months can lead to an additional expense of $100,000 for the sponsor. By addressing potential risks and discussing them upfront, sponsors can manage expectations and avoid unexpected charges.
As Orioson Swett Marden insightfully stated, 'There is no medicine like hope, no incentive so great and no tonic so powerful as expectations of something better than tomorrow.' This notion reinforces the importance of clarity and communication in the planning stages.
To make informed decisions, sponsors should focus on key elements in CRO cost proposals, including clarity of pricing and the identification of potential hidden fees. Furthermore, considering recent trends in CRO pricing can provide valuable context; for instance, many organizations are moving towards more fixed fee structures that enhance predictability in budgeting.
It is also crucial to examine case studies demonstrating the importance of proper planning during the RFP process. David Kim emphasized that identifying potential risks upfront can lead to better financial management and clarity regarding costs associated with delays or changes throughout the trial. Moreover, understanding the impact of medtech research studies on local economies—such as job creation, economic growth, and healthcare improvement—can further guide sponsors in selecting a CRO that aligns with their strategic goals.
Schedule Meetings and Ask Questions
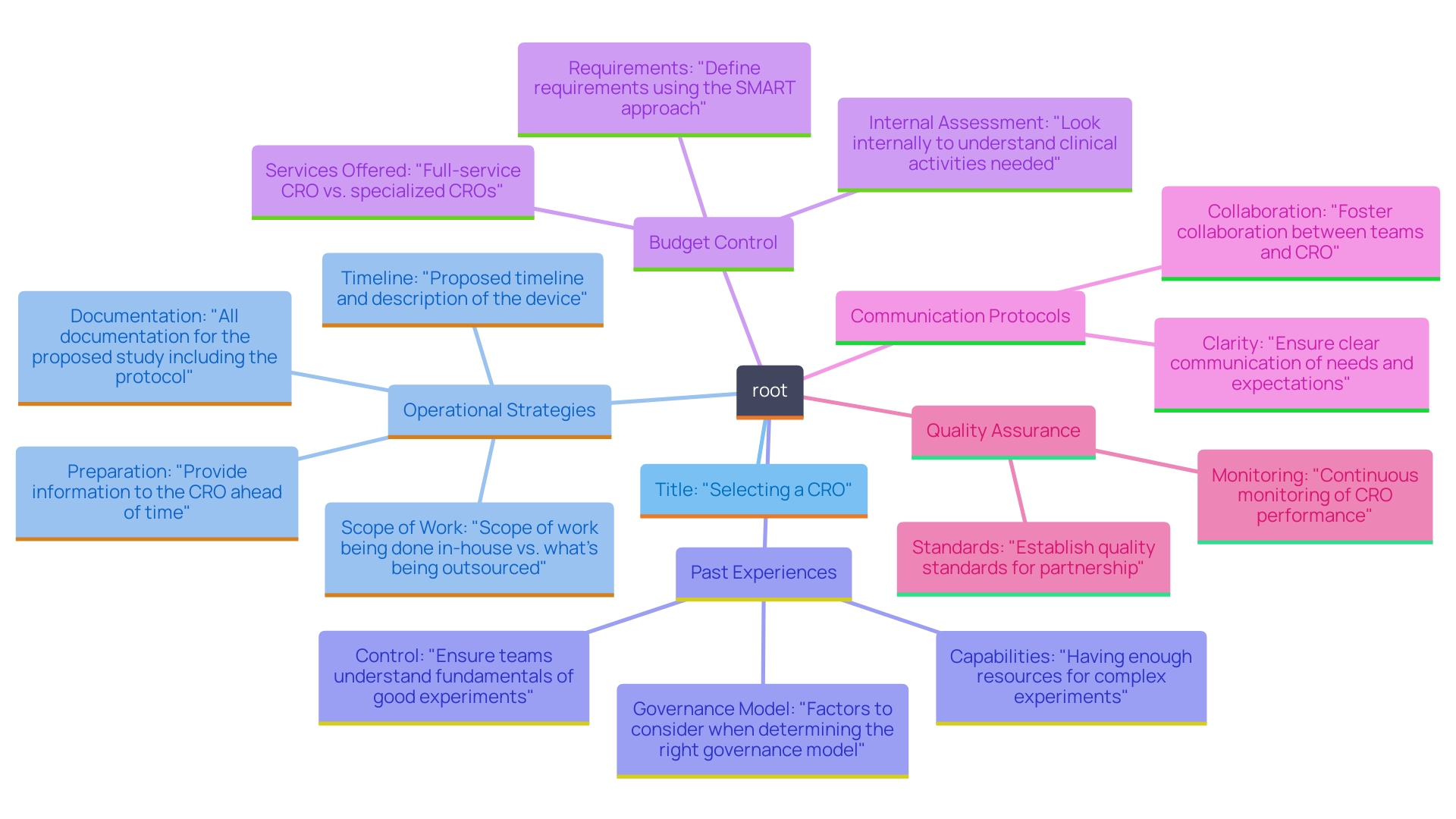
Arranging meetings with top candidates is a crucial step in selecting a Contract Research Organization (CRO) for your medical device project. As Patricio Ledesma, an expert in clinical studies, emphasizes, 'Effective communication during the selection process is key to establishing a successful partnership.' These discussions allow for a comprehensive understanding of how each CRO approaches project management, communication, and problem-solving methodologies. To maximize the effectiveness of these meetings, prepare a targeted list of questions that delve into their operational strategies.
Consider inquiring about their past experiences with similar projects, their process for managing timelines and budgets, and how they ensure quality control throughout the testing phase. Additionally, inquire about their communication protocols—specifically, how they intend to keep you informed of progress and address any issues that may arise.
By referencing successful case studies, such as a recent project where clear communication facilitated a 30% reduction in testing timelines, you can gain insights into effective practices. This interaction not only offers insights into their organizational culture but also uncovers their readiness to work together effectively—an essential element for the success of any research study. Engaging in this detailed dialogue can illuminate the potential for a fruitful partnership.
At bioaccess™, directed by CEO Julio Martinez-Clark and COO Monica Mora, we focus on providing extensive research management services customized for medical device firms aiming to maneuver through the intricacies of the Latin American market. Julio’s advocacy for Medtech research and Monica's operational expertise ensure that we provide accelerated study services, including Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies. By selecting bioaccess™, you collaborate with a vetted CRO that comprehends the distinct environment of Colombia and the prospects it offers for successful research studies.
Check References and Past Performance
Before finalizing any partnership with a Contract Research Organization (CRO), it is essential to thoroughly engage with the references they provide. Inquire specifically about their experiences, focusing on critical performance indicators such as the CRO's ability to adhere to timelines, manage budgets efficiently, and maintain clear and effective communication throughout the process. Research indicates that a well-executed reference check can significantly impact the selection of a CRO, often serving as a predictor of future performance. For example, research indicates that organizations that carefully verify references achieve a 30% greater success rate in studies compared to those that do not. This is backed by industry specialists, such as Dushyanth Surakanti, Founder and CEO of Sparta Biomedical, who shares insights from bioaccess® during its initial human study in Colombia, and Dr. John B. Simpson of Avinger, Inc., who discusses their favorable experience with OCT-guided atherectomy research in Cali, Colombia. Grasping these dynamics is essential for guaranteeing that the CRO you choose has a proven history of reliability and effectiveness in trials.
Moreover, embracing the uncertainties inherent in medical research is vital for success. By carefully vetting CRO capabilities and assessing their references, including the expertise offered by LATAM CRO Experts and insights from Julio G. Martinez-Clark on Regulatory Affairs and commercialization, you can mitigate this uncertainty. Thus, by evaluating references, you not only gather insights into a CRO's past performance but also equip yourself with the knowledge necessary to make informed choices in the ever-evolving field of research.
Finalize the Agreement
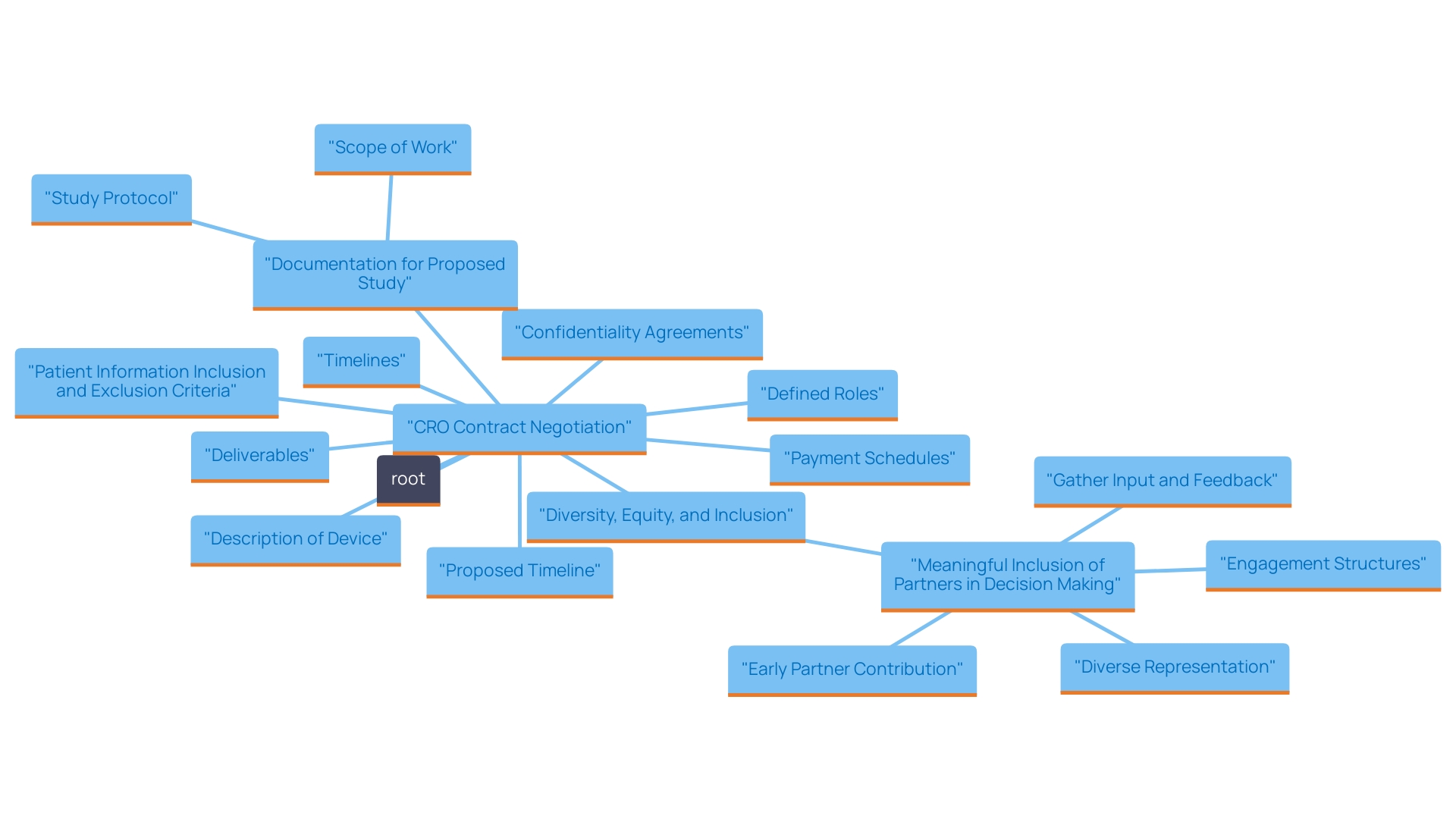
Selecting a Contract Research Organization (CRO) is just the beginning; the real challenge lies in negotiating the terms of the contract meticulously. It is imperative to ensure that every aspect of the collaboration is clearly articulated, including timelines, deliverables, payment schedules, and confidentiality agreements. A well-structured contract not only delineates the expectations of both parties but also serves as a foundation for a successful partnership.
For example, bioaccess® focuses on extensive trial management services that encompass feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting. Their expertise in early-feasibility, first-in-human, pilot, pivotal, and post-market follow-up studies enables expedited research study services in Latin America. This is particularly beneficial for medical device startups facing challenges such as regulatory hurdles, competition from established players, and recruitment issues.
Furthermore, Scimega's reputation for providing research start-up times that are 50% quicker than the North American average illustrates how effective negotiation and clear contract components can result in exceptional performance in studies. Their focus on maintaining high enrollment rates in global oncology studies highlights the benefits of efficient processes and well-negotiated contracts. By prioritizing clear communication and defined roles within the CRO agreement, organizations can significantly enhance their study performance and foster a collaborative environment conducive to success.
In preparing for negotiations, consider incorporating the following key elements into your CRO contract: * Clearly defined roles and responsibilities for both parties. * Detailed timelines that reflect realistic expectations, keeping in mind Colombia's expedited IRB/EC and MoH review process. * Comprehensive deliverables that outline what is expected at each project phase. * Transparent payment schedules that prevent misunderstandings. * Strong confidentiality agreements to protect sensitive information.
Furthermore, as legal experts emphasize, it is crucial to understand the nuances of negotiation to further enhance contract effectiveness. "A well-negotiated contract not only protects your interests but also fosters a collaborative spirit essential for clinical research success," notes legal professional [insert name].
Moreover, integrating considerations of diversity, equity, and inclusion into CRO agreements is becoming increasingly important. Organizations that prioritize these aspects can expect more innovative solutions and a broader range of perspectives, ultimately leading to enhanced results.
By addressing these elements, you can create a robust framework for your partnership that minimizes risks and maximizes the potential for successful outcomes in your medical device trials.
Conclusion
Selecting the right Contract Research Organization (CRO) is a critical decision that can significantly impact the success of medical device trials. This article has outlined the essential steps involved in this process, beginning with a clear definition of research needs. Understanding specific objectives, patient populations, and regulatory requirements lays the groundwork for effective collaboration with a CRO.
Researching and evaluating potential CROs based on their qualifications, experience, and compliance with regulatory standards is fundamental. By prioritizing organizations with proven track records, stakeholders can enhance their chances of successful trial outcomes. Furthermore, assessing proposals and comparing costs ensures that financial considerations align with the value offered by each CRO.
Engaging in meaningful discussions with top candidates allows for a deeper understanding of their methodologies and operational strategies. Checking references and past performance provides insights into their reliability and effectiveness, reinforcing the importance of thorough due diligence. Finally, finalizing a well-structured agreement that clearly delineates expectations and responsibilities is paramount to fostering a collaborative environment.
In conclusion, navigating the complexities of medical device trials requires a systematic approach to selecting a CRO. By following these outlined steps, stakeholders can not only enhance the likelihood of successful trials but also contribute to the advancement of healthcare innovation. The commitment to patient-centric outcomes and regulatory compliance remains essential in ensuring the integrity and success of clinical research endeavors.




