Introduction
In the evolving landscape of clinical research, Paraguay emerges as a promising location for medical device trials, characterized by a supportive regulatory framework and a diverse patient population. As the National Directorate of Medicines and Food (DINACOM) enhances its oversight, the potential for innovative research grows, attracting attention from U.S. MedTech companies seeking to leverage the region's unique advantages. This article delves into the critical aspects of conducting clinical trials in Paraguay, from selecting the right Contract Research Organization (CRO) to establishing effective communication and monitoring performance.
By understanding these elements, stakeholders can navigate the complexities of clinical research and contribute to the advancement of healthcare in Latin America.
Understanding the Landscape of Medical Device Trials in Paraguay
Paraguay provides a compelling environment for conducting medical device studies, marked by an increasing focus on clinical research and a favorable regulatory framework overseen by the National Directorate of Medicines and Food (DINACOM). This ensures adherence to stringent standards and regulatory compliance reviews. A key advantage of carrying out studies in Paraguay is its diverse patient population, which facilitates the collection of comprehensive and varied data. Engaging with local practices and building relationships with regional stakeholders can significantly streamline processes and enhance patient recruitment.
For example, Urotronic's first-in-human (FIH) study of the Optilume drug-coated balloon in Panama illustrates successful medical investigation in the area, tackling recurring urethral strictures in men. This underscores the trend of U.S. MedTech firms utilizing Latin American environments for their studies, emphasizing the region's increasing significance in global medical investigations.
Furthermore, contemporary subjects like artificial intelligence and translational studies are gaining popularity in the setting of medical evaluations in Paraguay. These innovative approaches can enhance data analysis and improve patient outcomes, making the region increasingly attractive for investment. The successful treatment of eleven patients with chronic low back pain using ReGelTec's HYDRAFIL™ in Colombia further illustrates the impact of innovative medical devices on patient care. Prioritizing studies on health determinants will be crucial in attracting further investment and supporting comprehensive health improvements in Paraguay.
Anticipated regulatory updates in 2024 are expected to strengthen this framework, promoting external sponsorship and ensuring robust regulatory oversight. Dr. Adrian Ebner, head of endovascular and cardiovascular surgery at the Italian Hospital in Asuncion, Paraguay, highlighted the importance of these advancements, mentioning that the successful conclusion of Urotronic's FIH study marks a milestone in regional clinical research. He highlighted that with a median attribution ratio for all-grade adverse events in placebo arms at 53.9%, understanding these factors is essential for ensuring successful study outcomes in Paraguay.
Key Considerations for Choosing the Right CRO for Your Trials
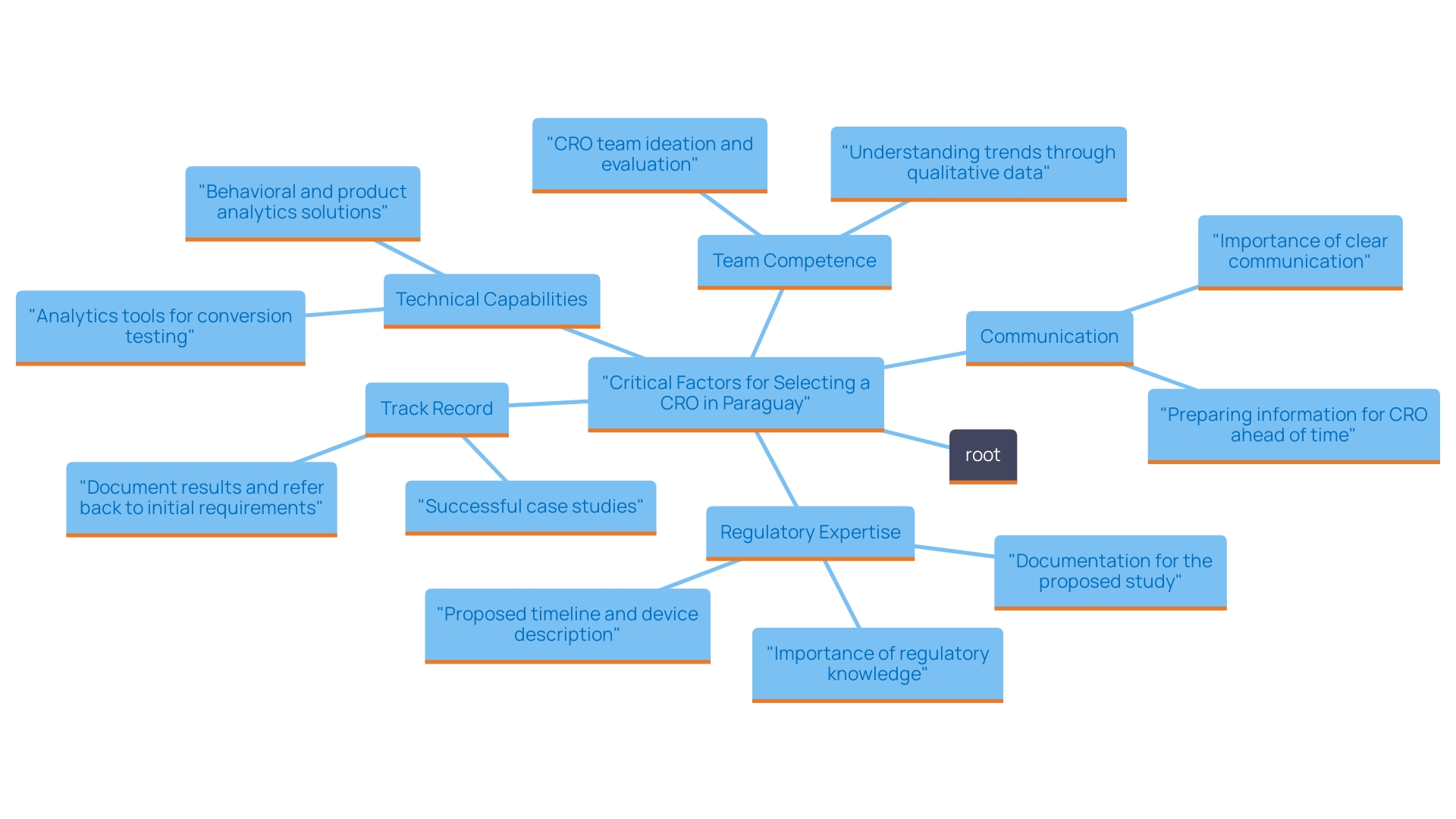
When choosing a CRO for medical device trials in Paraguay, consider the following key factors:
-
Regulatory Expertise: Navigating local regulations is crucial. A CRO with a deep understanding of the approval process in Paraguay and prior experience in the region will be highly advantageous. For instance, bioaccess® has established itself as a vetted CRO and consulting partner for U.S. medical device companies in Colombia, showcasing its regulatory expertise. As emphasized by Dr. Adrian Ebner, director of endovascular and cardiovascular surgery at the Italian Hospital in Asuncion, Paraguay, "Urotronic, Inc. announced in 2022 that it successfully completed the FIH of its Optilume drug-coated balloon (DCB) in men with recurrent urethral strictures in Panama," which underscores the critical role of regulatory knowledge in achieving successful study outcomes.
-
Track Record: Examining the CRO's history of successful experiments, particularly in the medical device sector, is essential. Seek testimonials or case studies, like Urotronic's FIH study of the Optilume DCB in Panama, which highlights their effectiveness in managing complex evaluations and contributing to the advancement of medical research in Latin America. Bioaccess® specializes in various studies, including Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies, ensuring a comprehensive approach to clinical research.
-
Technical Capabilities: Evaluate the CRO's resources and technological infrastructure. Ensure they have robust data management systems and state-of-the-art facilities for conducting experiments. These capabilities are vital for ensuring the accuracy and reliability of experimental results.
-
Team Competence: The qualifications and experience of the CRO’s team members are critical. For example, Juan Cuya, MD, is a Clinical Trial Associate with expertise in Regulatory Affairs and product launches, while Dr. Sergio Alvarado focuses on innovative medical research and artificial intelligence in Latin America. A knowledgeable and skilled team can significantly influence the success of the endeavor, ensuring meticulous planning and execution.
-
Communication: Effective communication is key to a successful collaboration. Ensure the CRO can provide timely updates and maintain transparency throughout the study process. This fosters trust and ensures that all stakeholders are well-informed.
By thoughtfully evaluating these factors, you can choose a CRO that fits your project’s needs and improves your likelihood of success in the study. With Paraguay's urbanization rate at 61.6%, understanding the local context and utilizing experienced CROs like bioaccess® can lead to more efficient and effective studies. This is particularly relevant in light of the lower urbanization rate, which suggests a need for tailored approaches to CRO selection that consider regional healthcare dynamics. In summary, emphasizing regulatory knowledge, established track records, technical skills, team proficiency, and effective communication will greatly improve the chances of successful studies.
Establishing Clear Communication with Your Chosen CRO
Once a CRO is chosen, establishing clear communication protocols becomes paramount in the context of comprehensive clinical study management services. This process begins with defining structured communication channels, including regular meetings and updates, and identifying designated points of contact on both sides to streamline communication and ensure accountability. Utilizing project management tools such as Asana or Trello can help in tracking progress and maintaining transparency, allowing both parties to stay aligned on objectives and timelines.
Effective communication is not only about frequency but also quality. As Aidan Gannon, Head of Innovation and Client Services at Longboat Clinical, emphasizes, "We don’t make interfaces easy to use or beautiful in many cases. We need, as an industry, to improve on that. No one wants to use an interface that they can’t understand or that just doesn’t appeal to them visually, so we need to be better at that." This quote underlines the necessity for intuitive communication tools that enhance user experience and facilitate better interactions.
The case study titled 'Unlocking Benefits of Patient-Centred Trials' emphasizes the significance of effectively managed communication at every stage to attain genuine patient-centricity and enhanced study outcomes. In decentralized studies, where around 4% of research is completely virtual, Nuala Ryan highlights the necessity for consistent, human-to-human interaction with patients. This emphasizes the significance of keeping open channels of communication not only for project management but also for compliance evaluations and the management of import permits, which are essential for the success of research studies.
Encouraging open dialogue is essential to promptly address any concerns or challenges. By fostering a collaborative environment and employing tools like Slack for real-time communication, efficiency and effectiveness can be significantly enhanced. Implementing best practices for communication in research studies, such as regular check-ins and clear updates, guarantees a strong partnership with CROs and a more efficient process, ultimately promoting global health enhancement through international collaboration and innovation in Medtech.
Monitoring and Evaluating the CRO's Performance
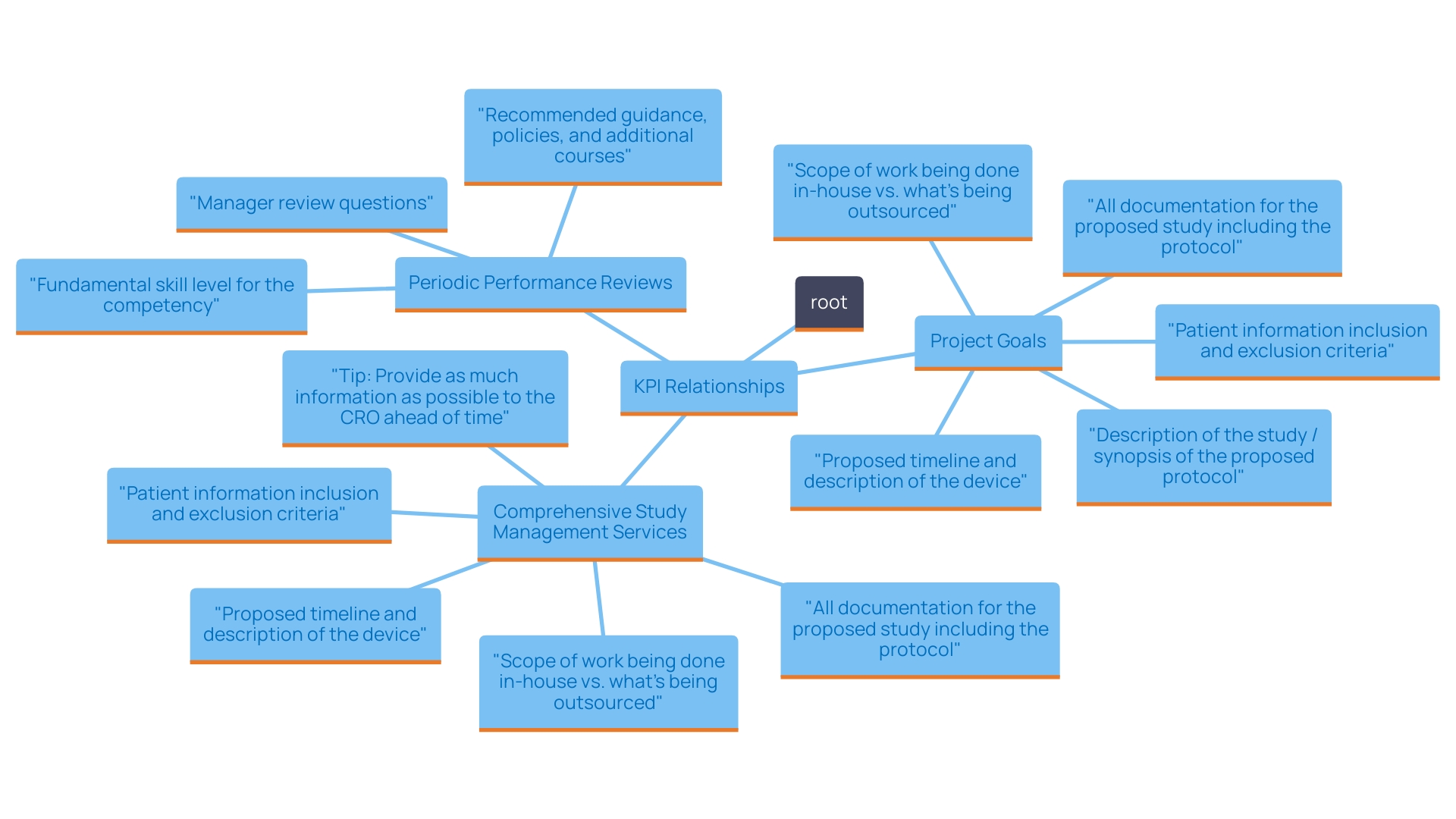
To effectively monitor and evaluate your Clinical Research Organization (CRO), it is critical to establish key performance indicators (KPIs) that align directly with your project goals. These KPIs should include metrics pertinent to the CRO's performance, ensuring they effectively enhance the success of your medical initiatives.
Our comprehensive study management services encompass:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Setup
- Import permits
- Project oversight
- Reporting
This ensures meticulous management of all aspects of your medical investigation. Regularly reviewing progress against these KPIs is essential for maintaining oversight and ensuring the CRO's strategies are effective.
In addition to tracking performance metrics, it is vital to schedule periodic performance reviews that assess the CRO's adherence to:
- Timelines
- Budgets
- Industry regulations
Specific aspects to review should include:
- The accuracy of patient recruitment timelines
- The efficiency of data collection processes
- Adherence to ethical guidelines
Utilizing feedback from your team is also essential in gauging the CRO's effectiveness. By maintaining diligent oversight through these methods, you can ensure that your research study remains on track, meets the highest standards of excellence, and contributes positively to local economies through job creation and improved healthcare.
Building Long-term Partnerships with CROs
Establishing and nurturing long-term partnerships with Contract Research Organizations (CROs) from the very beginning is crucial for successful collaborations. At bioaccess®, our comprehensive research project management services encompass:
- Feasibility studies
- Site selection
- Compliance reviews
- Setup
- Import permits
- Project oversight
- Reporting
This ensures that all aspects of the research process are meticulously handled. Continuous communication that extends beyond trial-specific matters is essential; sharing industry insights and updates can keep both parties aligned with current trends and practices. Additionally, collaborating on future projects can further solidify these relationships.
A retrospective analysis after the completion of a study serves as a valuable tool in assessing the success of the sponsor-CRO collaboration. This process involves evaluating timelines, identifying problems, and reviewing adherence to protocols. As Katrina Petrosino, a seasoned Project Manager, notes, "Following completion of a study, doing a retrospective analysis can help to answer the following questions: Were timelines met?"
A case study titled 'Assessing Success in Sponsor-CRO Collaboration' highlights how post-study analyses provide critical insights, allowing for the identification of areas needing improvement and enhancing future collaborations. This methodical evaluation can pinpoint deviations and data entry issues, which can be mitigated through study-specific training on the data entry system, rather than relying solely on generic training. Recent statistics indicate that organizations engaging in rigorous retrospective analyses have reported a 30% increase in collaboration success rates, underscoring the importance of this practice.
Recognizing the contributions of the CRO and celebrating shared successes also play a pivotal role in fostering a robust partnership. By focusing on relationship-building initiatives and ensuring open, consistent communication, both sponsors and CROs can gain from enhanced collaboration, resulting in more successful trials and long-term success. Furthermore, through our Medtech clinical studies in Latin America, we create ripples of positive impact on local economies, enhancing job creation, promoting economic growth, and improving healthcare systems while fostering international collaboration.
Conclusion
Paraguay stands out as a burgeoning hub for clinical trials involving medical devices, offering a unique combination of a supportive regulatory environment and a diverse patient demographic. The insights gathered throughout this article underscore the importance of selecting the right Contract Research Organization (CRO) and establishing clear communication pathways. These factors are pivotal for navigating the complexities inherent in clinical research and ensuring that trials are conducted efficiently and ethically.
The article emphasizes the critical role of regulatory expertise, a proven track record, and technical capabilities when choosing a CRO. Such considerations not only enhance the likelihood of successful trial outcomes but also contribute to the overall advancement of healthcare in the region. Moreover, establishing effective monitoring and evaluation mechanisms is essential for maintaining oversight and ensuring that CROs adhere to timelines and regulatory standards.
Ultimately, the potential for innovation and growth in Paraguay’s clinical research landscape is significant. By fostering long-term partnerships with CROs and prioritizing effective communication, stakeholders can drive advancements in medical technology that benefit both local populations and the global healthcare community. As the landscape continues to evolve, Paraguay is well-positioned to play a crucial role in the future of medical device trials, paving the way for enhanced patient care and improved health outcomes across Latin America.




