Introduction
Navigating the complexities of medical device trials demands a strategic approach, particularly when selecting a Contract Research Organization (CRO) that aligns with specific project needs. As the landscape of clinical research evolves, understanding the critical factors that influence trial success becomes paramount. From assessing research requirements and conducting market research on potential CROs to evaluating their experience and ensuring compliance with regulatory standards, each step plays a vital role in shaping the outcome of a trial.
This article delves into the essential considerations for selecting a CRO, highlighting best practices and insights that can enhance the effectiveness of medical device trials. By fostering informed decision-making, stakeholders can pave the way for successful partnerships that not only meet regulatory expectations but also drive innovation in healthcare.
Assess Your Research Needs
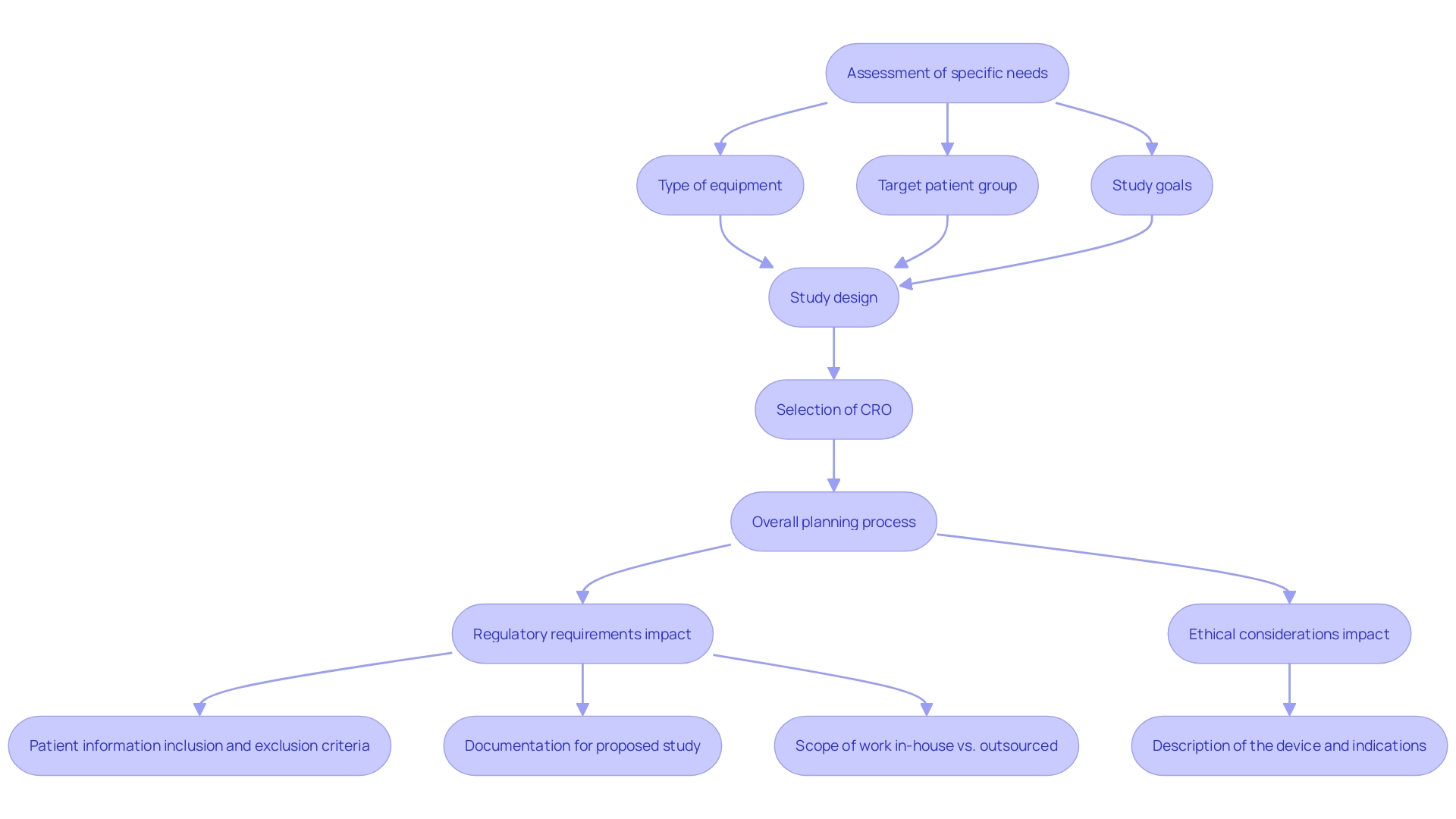
To commence a successful medical apparatus test, it is crucial to delineate its specific needs clearly. This starts with assessing essential elements such as the kind of equipment being examined, the targeted patient group, and the goals of the study. Comprehending these elements guides the study design and assists in recognizing the expertise required from a Contract Research Organization (CRO).
Statistics indicate that roughly 30% of medical equipment studies encounter considerable delays due to ambiguous requirements or insufficient planning (Source: [insert source]). The intricacy of the apparatus may determine the extent of regulatory examination needed, affecting both the study's framework and length. Additionally, the target population—whether children, adults, or individuals with specific health conditions—determines ethical considerations, especially given recent guidelines advocating for minimal risk and direct benefits in research involving vulnerable groups.
Aligning the study's objectives with the selection of a CRO is crucial. A CRO experienced in your device category can provide valuable insights into regulatory requirements and best practices, ensuring a streamlined approach that enhances the study's efficacy and ethical standing. Our services include feasibility and selection of research locations, investigator selection, compliance reviews, study setup, import permits, and comprehensive project management and reporting. For example, the case study on financial transparency in medical research emphasizes the significance of revealing funding sources and possible conflicts of interest, crucial for upholding ethical standards throughout the research process. By thoroughly evaluating these requirements and employing customized solutions for medical product success, you can cultivate a cooperative relationship with your CRO, paving the way for a successful and ethically sound study.
Conduct Market Research on CROs
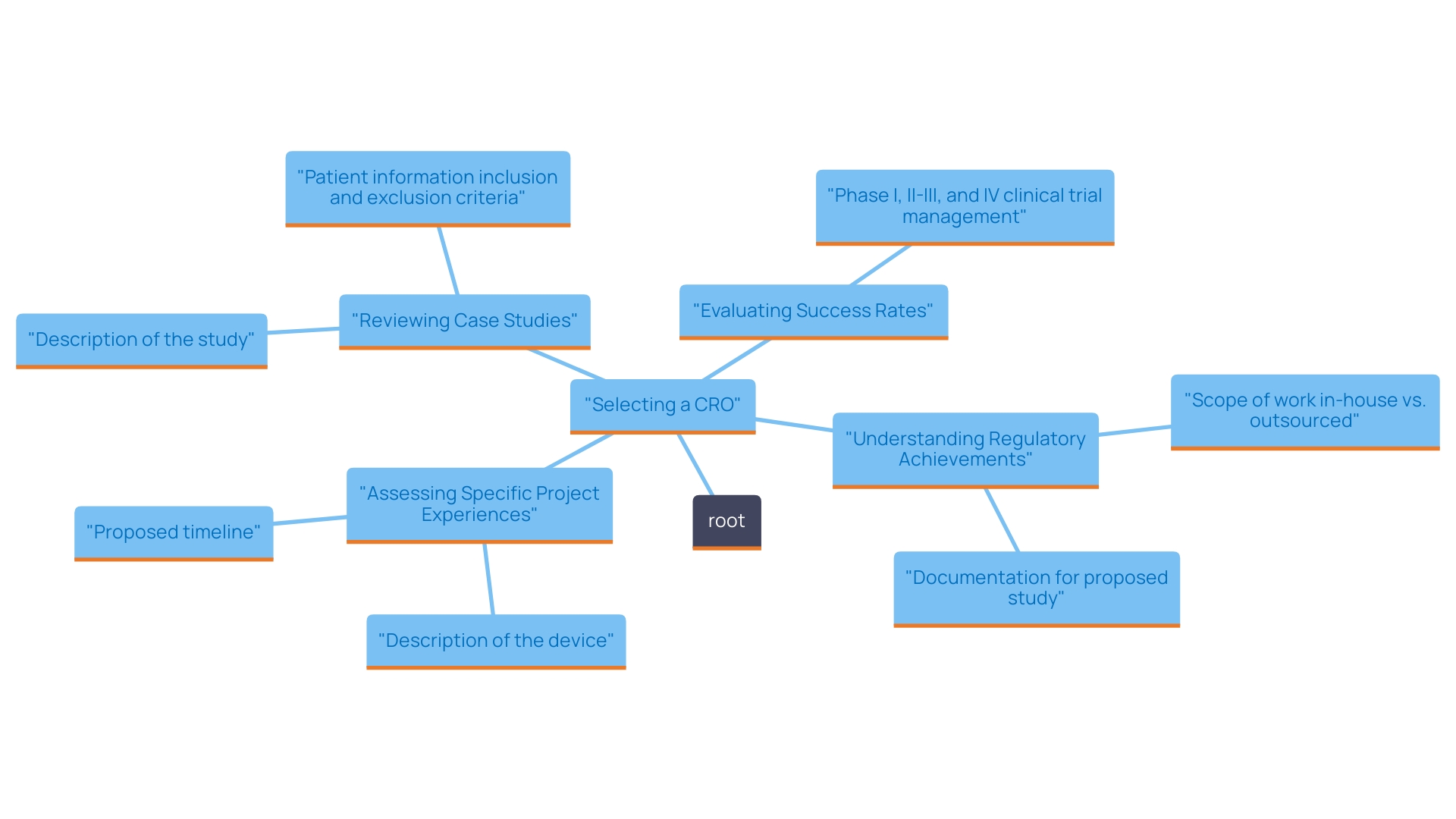
Carrying out comprehensive investigation on Contract Research Organizations (CROs) functioning in the Dominican Republic is crucial for guaranteeing successful medical equipment studies. It is essential to utilize a range of online resources, including industry publications, academic journals, and professional networks, to identify CROss with a proven track record in comprehensive clinical study management services, such as feasibility studies, site selection, compliance reviews, and setup. One effective method is to compile a list of potential CROss based on their historical performance in medical device studies. For instance, case studies from previous projects can provide insights into their effectiveness, showcasing success stories that highlight their expertise in early-feasibility and pivotal studies. Notably, organizations that have conducted extensive market research have demonstrated significant success in optimizing results. It is also advantageous to seek referrals from colleagues in the research field to gather firsthand accounts and recommendations. According to Pamela Bump, Content Growth Team Manager at HubSpot, 'What works for one business may not work for all.' This underscores the necessity of tailoring your selection process to align with your specific project needs and goals, particularly in the unique context of the Dominican Republic. Moreover, recent market research statistics suggest that about 65% of CROss in the Dominican Republic are currently adopting innovative methods to improve efficiency in studies, a trend that is especially pertinent considering the rising demand for medical equipment studies. Among the top CROs in the Dominican Republic for 2024 are XYZ CRO, ABC Trials, and MNO Research, which have all received positive evaluations based on their successful project outcomes and client satisfaction. By systematically assessing potential CROss through these channels and concentrating on those with proficiency in accelerated medical product clinical studies, you will be better positioned to choose a partner that not only meets regulatory requirements but also aligns with your strategic goals, ultimately improving the success of your clinical evaluations.

Evaluate CRO Experience and Expertise
When assessing a Contract Research Organization (CRO) for your medical product studies, reviewing their experience with comparable projects is crucial. Start by reviewing their case studies, which provide concrete examples of past performance and outcomes. For example, ReGelTec's Early Feasibility Study on HYDRAFIL™ effectively treated eleven individuals suffering from chronic low back pain in Colombia, demonstrating the CRO's capacity to manage the intricacies of medical product evaluations. Likewise, Flow-FX's first-in-human study of the Flow-Screw device for intraosseous antibiotic delivery demonstrates their effectiveness in meeting regulatory requirements. Moreover, GlobalCare Clinical Studies' partnership with bioaccess™ has led to an impressive decrease in subject recruitment time by over 50% and a retention rate surpassing 95%. These successful case studies not only demonstrate these accomplishments but also highlight the CRO's ability to manage your specific study effectively. Statistics show that top CROs have attained success rates of up to 85% in regulatory submissions, which is vital considering the changing regulatory environment for medical products since the European Commission's updates in 2017, which established more rigorous requirements for evidence and market entry. As a result, CROss have increasingly taken on pivotal roles, providing scientific evidence and regulatory assistance to ensure compliance with these guidelines. Furthermore, evaluate any pertinent regulatory approvals that the CRO has secured, as this information can offer insight into their ability to manage your specific study effectively. According to an expert in the field, 'A thorough evaluation of a CRO’s experience, including their historical success rates and regulatory achievements, is vital in ensuring the selection of a partner that can deliver quality results.' By merging this information, stakeholders can acquire a thorough understanding of a CRO's capabilities, assisting in achieving successful results in studies for medical devices.
Assess Compliance with Regulatory Standards
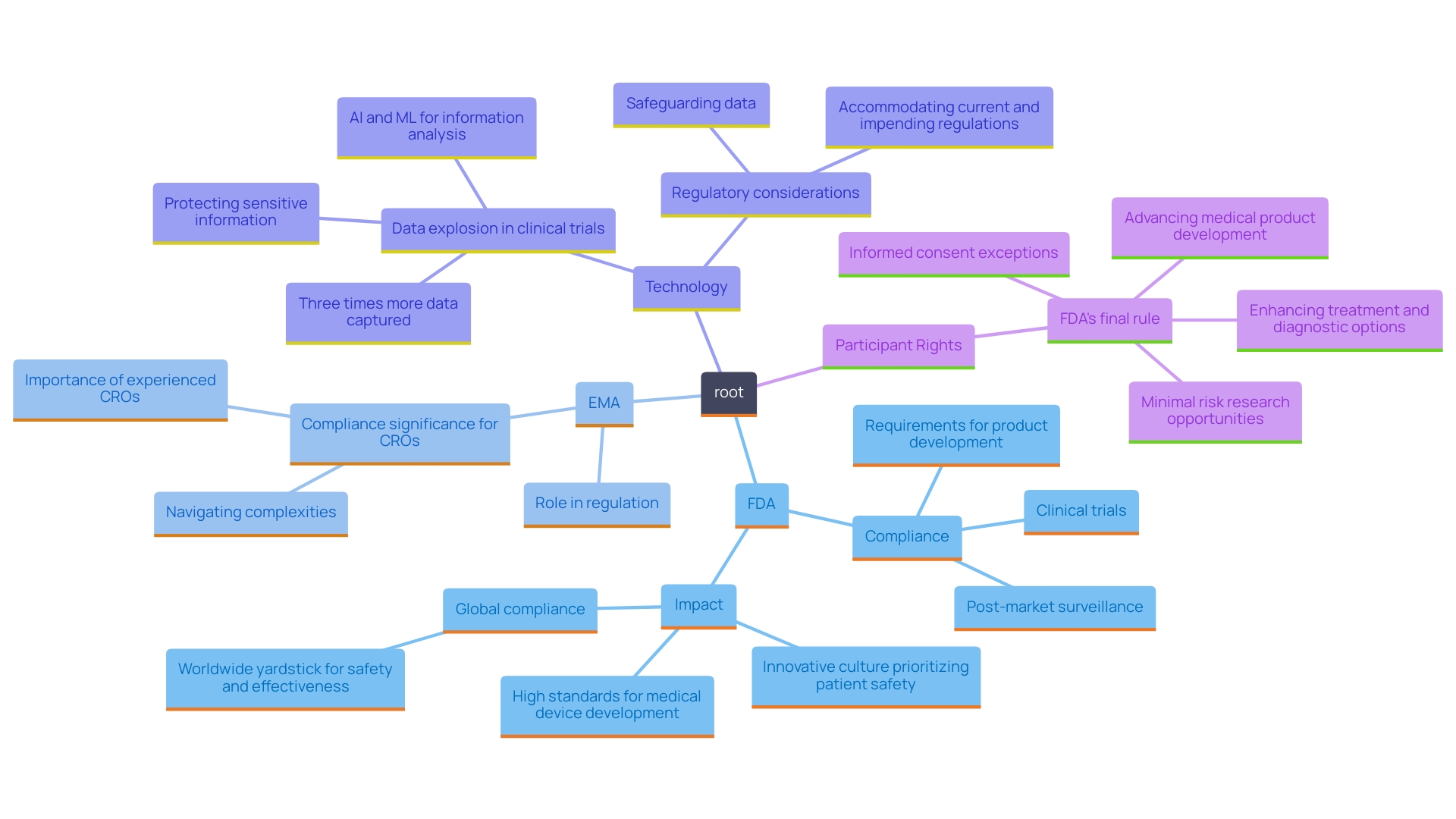
Making certain that your Contract Research Organization (CRO) adheres to both local and international regulatory standards is crucial for the success of any study. This entails a thorough understanding of the regulations established by pivotal authorities, including the U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA), and corresponding regulatory bodies in the Dominican Republic. According to recent statistics, adherence to these regulations can enhance the likelihood of success by up to 30%, underscoring the importance of compliance.
Compliance with these regulations is not merely a formality; it serves as a critical foundation for advancing clinical research. A CRO well-versed in these guidelines can significantly enhance the integrity of your study, mitigate risks, and promote adherence to best practices. As the pharmaceutical, biotechnology, and medical device industries increasingly rely on CROss to navigate complex regulatory landscapes, the necessity for such compliance cannot be overstated.
Recent advancements in technology, such as data analytics and electronic health records, have further transformed regulatory compliance processes within clinical studies. For instance, a case study on bioaccess®, a leading Medtech CRO in Latin America, demonstrated a 25% increase in compliance tracking efficiency through the implementation of electronic health records. This innovation not only improves data tracking and compliance monitoring but also enhances overall study efficiency and reliability.
Katherine Ruiz, an expert in Regulatory Affairs for medical devices and in vitro diagnostics in Colombia, emphasizes the importance of partnering with knowledgeable CROss. Her insights emphasize how adherence to specific regulatory requirements in the Dominican Republic, including thorough documentation and regular audits, is essential for successful navigation.
By collaborating with experienced CROs like bioaccess®, you can streamline your clinical study processes while ensuring adherence to essential regulatory standards, thereby contributing to the successful development of innovative therapies.
Review Financial Considerations
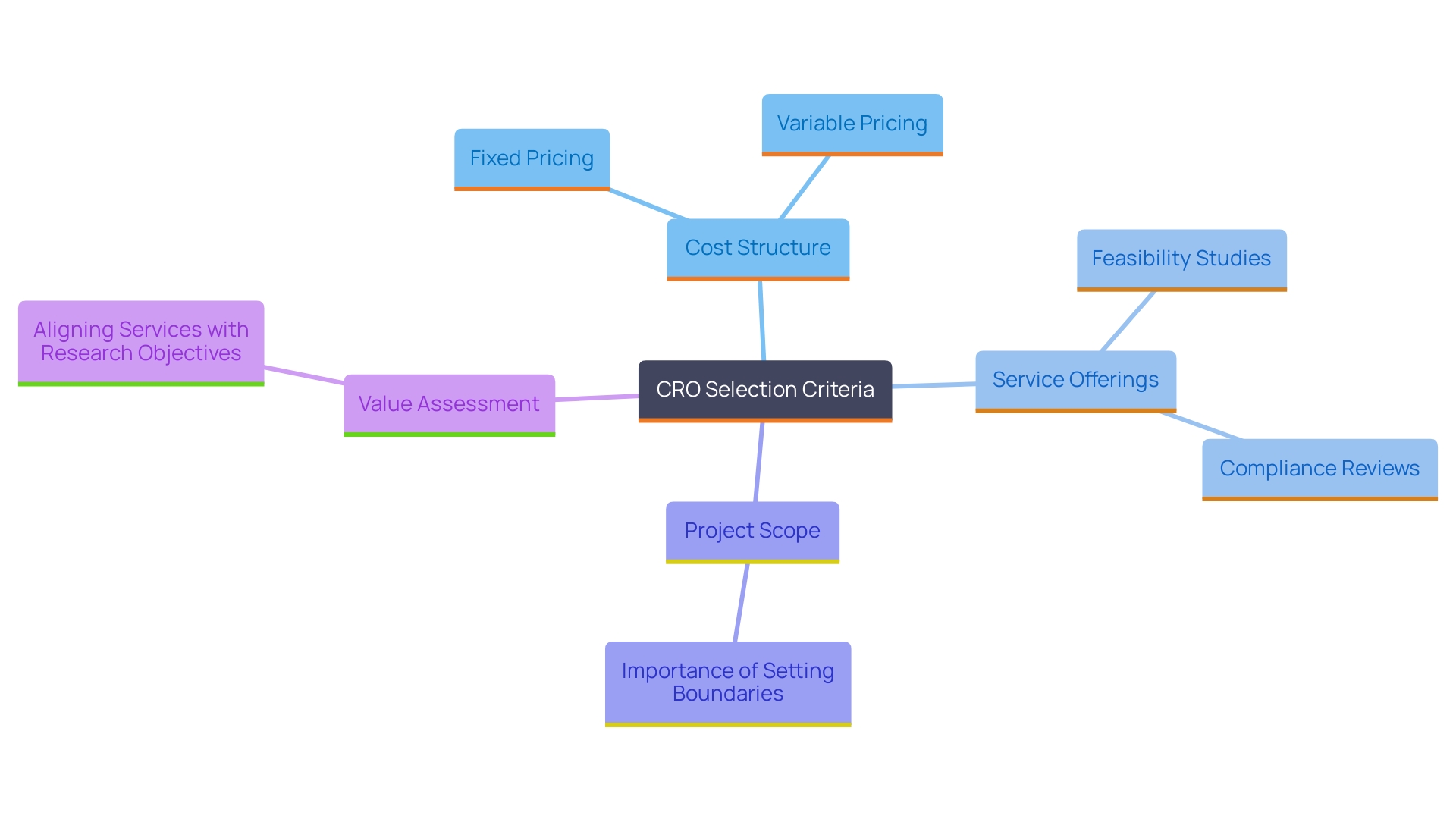
When choosing a Contract Research Organization (CRO) for medical device studies, it is essential to request detailed proposals from your shortlisted candidates. These proposals should include a comprehensive breakdown of all costs associated with the study, highlighting not just the financial requirements but also the range of services provided, such as feasibility studies, site selection, compliance reviews, setup, import permits, project management, and reporting. For instance, a comparison might show that while some CROss offer fixed pricing models, others may present variable costs that fluctuate based on the number of tests conducted or additional services requested.
With some freelancers charging as low as $20 per hour for CRO services, it is essential to compare these proposals meticulously. The assessment should go beyond mere pricing; you must also consider the value of services offered by each CRO. Top-tier agencies often charge premium rates reflective of their extensive experience and proven track record, while lower-tier agencies may present more attractive price points at the cost of service quality.
Lucia van den Brink from Speero emphasizes the importance of maintaining boundaries in project scope: "Working with retainers can give some space to breathe, but also: if you don’t set boundaries (i.e. number of tests between 1 – 3 per month for example) you and the team can easily over-deliver." Integrating this perspective is particularly relevant when evaluating budget constraints and ensuring that your CRO can effectively meet your research needs without exceeding financial limits.
In addition to price comparisons, it is vital to consider the financial implications of CRO proposals—particularly in terms of how they align with your research objectives and budget. Considering different financial factors, such as cost structure and the total value provided, can greatly influence the success of your study. By aligning your budgetary expectations with a CRO's capabilities and service offerings, including their expertise in early-feasibility, first-in-human, pilot, pivotal, and post-market follow-up studies in Latin America, you can make a more informed decision that balances cost with value. Ultimately, grasping the subtleties of cost versus value in CRO services will enable you to select a partner that not only satisfies your financial requirements but also aids your research objectives efficiently, as highlighted by industry authorities such as Julio G. Martinez-Clark.
Conduct Interviews and Assess Compatibility
To ensure a successful partnership with a Contract Research Organization (CRO), it is essential to arrange meetings with key personnel from the CROss under consideration. During these discussions, focus on their method to research tests, including feasibility studies, site selection, and compliance reviews, as well as their communication strategies, which are essential for promoting effective collaboration. As highlighted by Hughes RG, communication failures have historically been a leading cause of sentinel events in clinical settings.
During the meetings, inquire about how the CRO manages challenges that may arise throughout the testing process. This not only provides insight into their operational capabilities but also allows you to assess their compatibility with your team. Evaluate their willingness to collaborate effectively and address any concerns proactively.
Employ structured interview questions such as: - Can you provide examples of past challenges encountered during trials and how your team addressed them? - What strategies do you implement to ensure clear communication among stakeholders throughout the process? - How does your organization measure the success of communication during a clinical study?
Additionally, inquire about their experience with Regulatory Affairs, as understanding how they navigate compliance requirements is vital for successful outcomes. This approach will aid in determining whether the CRO’s operational philosophy aligns with your research objectives, ultimately setting the foundation for a productive and synergistic relationship. Moreover, think about examining statistics showing that organizations with effective communication strategies tend to have a 30% higher success rate in reaching milestones, emphasizing the significance of clear and proactive communication.
Make Your Decision and Formalize the Partnership
Choosing the appropriate Contract Research Organization (CRO) for your medical equipment study is crucial for guaranteeing efficient project execution. After evaluating various candidates based on their expertise, reputation, and alignment with your objectives, it is essential to formalize the partnership through comprehensive contracts and agreements. These documents should clearly delineate the scope of work, establish timelines, and specify deliverables to avoid any operational ambiguities. CROs can aid in the feasibility and choice of research locations, as well as offer essential support in navigating the complex regulatory demands that medical device studies frequently encounter.
Tyler Younger, Director of Proposals at PharPoint, emphasizes the importance of transparency in this process: 'With every proposal, we focus heavily on providing a transparent overview of what partnering with PharPoint would be like – what our high-level strategy would be, what kind of experience the study team you’d be working with has, and of course, a budget specifically tailored to your requests with clear assumptions.' This level of clarity is crucial in fostering trust and ensuring that both parties are aligned on expectations.
Establishing empowering partnerships is often a precursor to successful development projects, especially for startups that may struggle with limited financial resources and disinterested research sites. Tailoring the partnership to fit the size and operational scale of your organization is advisable. For instance, larger biotech and pharmaceutical companies typically have well-defined internal processes that allow for outsourcing tasks with minimal collaboration. In contrast, smaller companies often encounter challenges due to rapid changes in staffing and planning. By ensuring that the partnership is suited to your company’s specific dynamics—such as having dedicated resources for key development drivers—you can mitigate operational gaps and facilitate a smoother progression through research trials.
When finalizing contracts with CROs, meticulous negotiation is essential. This process includes contract negotiation tips and best practices to formalize agreements effectively, ensuring that all stakeholders are aligned. For example, a study revealed that 75% of successful CRO partnerships stem from clearly defined roles and responsibilities within the contract; providing a source for this statistic will enhance its credibility. Ultimately, summarizing these key takeaways—such as the importance of transparency, tailored partnerships, and thorough contract negotiation—will set a solid foundation as you advance in your clinical research endeavors.
Conclusion
Selecting the right Contract Research Organization (CRO) is a critical step in ensuring the success of medical device trials. The process begins with a thorough assessment of research needs, which includes defining the specific requirements of the trial, understanding patient demographics, and aligning objectives with CRO expertise. Conducting comprehensive market research on potential CROs allows stakeholders to identify organizations with a proven track record in clinical trial management, enhancing the likelihood of achieving favorable outcomes.
Evaluating the experience and expertise of a CRO is essential, as demonstrated by successful case studies that highlight their capabilities in navigating regulatory complexities. Adherence to compliance standards is also paramount, as it forms the foundation for maintaining trial integrity and mitigating risks. Financial considerations play a significant role in the selection process, necessitating a detailed evaluation of proposals to ensure that services align with budgetary constraints while delivering value.
Establishing a strong partnership hinges on effective communication and compatibility. Engaging in interviews with key personnel from CROs allows stakeholders to gauge their operational philosophies and problem-solving approaches. Ultimately, formalizing the partnership through clear contracts and agreements sets the stage for a collaborative relationship that can adapt to the unique challenges of clinical trials.
The pathway to selecting a CRO is multifaceted, requiring diligence and strategic planning at every stage. By prioritizing informed decision-making and fostering transparent partnerships, stakeholders can enhance the efficacy of medical device trials, driving innovation and improving health outcomes in the process.




