Introduction
The evolution of Electronic Data Capture (EDC) systems marks a significant advancement in the realm of clinical trials, revolutionizing the way data is collected, managed, and analyzed. As the reliance on technology intensifies within the clinical research landscape, EDC systems emerge as indispensable tools that not only streamline data processes but also enhance the accuracy and integrity of trial outcomes.
This article delves into the fundamental aspects of EDC systems, exploring their key features, benefits, and the essential criteria for selecting the right system. Additionally, it addresses the challenges faced during implementation and highlights emerging trends that are shaping the future of data capture in clinical research.
By understanding the intricacies of EDC systems, professionals can better navigate the complexities of clinical trials, ultimately fostering improved healthcare outcomes and economic growth.
Understanding Electronic Data Capture (EDC) Systems
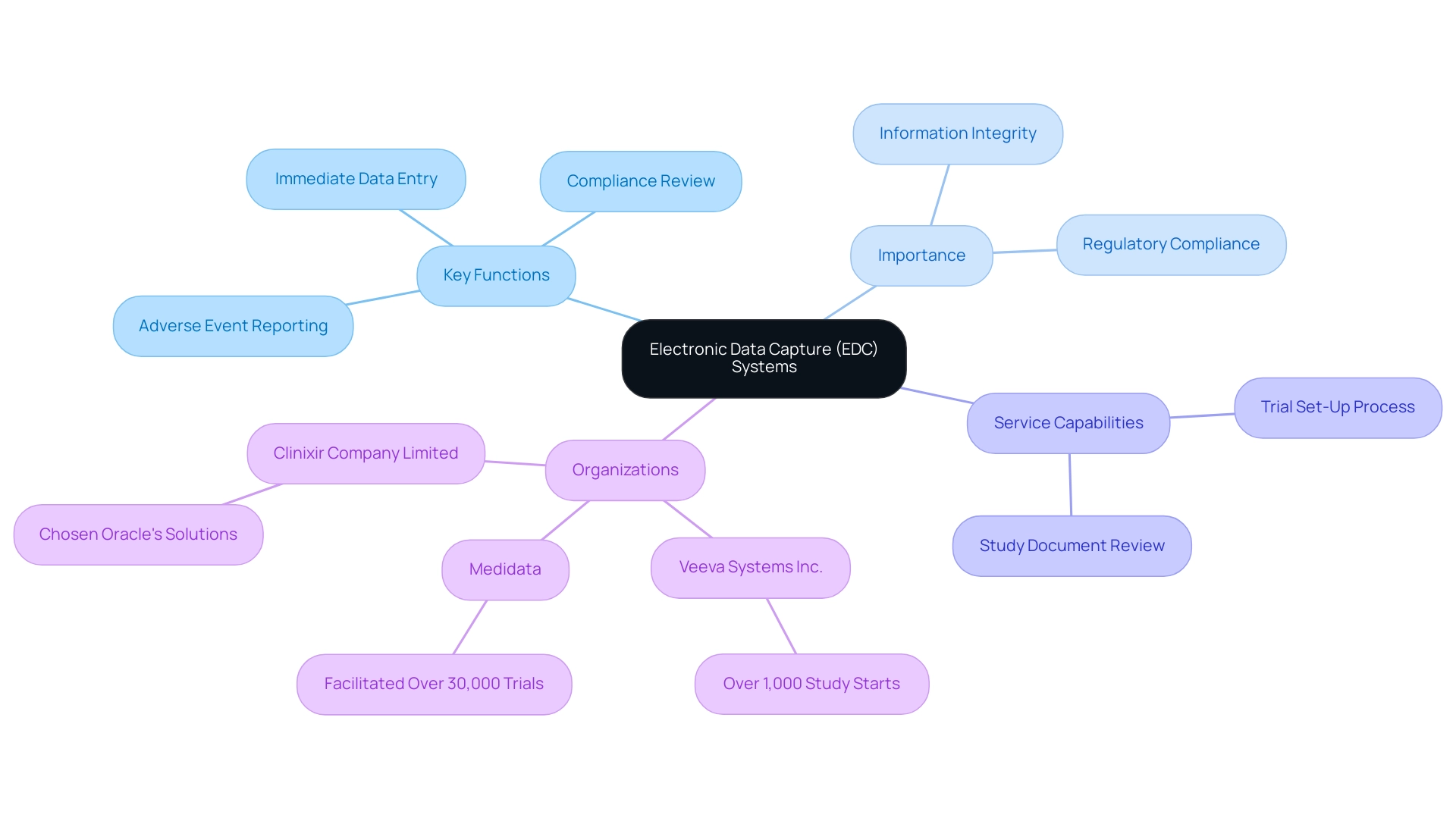
Electronic Data Capture (EDC) platforms signify a transformative advancement in the manner trial information is gathered, handled, and preserved, serving a vital function in the management of EDC clinical study services. These advanced software solutions progress past conventional paper-based methods, providing streamlined processes that improve both accuracy and accessibility. With features for immediate information entry and oversight, EDC platforms enable researchers to assess details swiftly, facilitating informed choices throughout the EDC clinical study lifecycle.
They facilitate specific service capabilities, such as:
- The review and feedback on study documents to ensure compliance with country requirements
- The trial set-up process
- The reporting of serious and non-serious adverse events
The importance of these frameworks in an EDC clinical study cannot be overstated; they are essential for preserving information integrity and ensuring adherence to regulatory standards. Organizations such as Veeva Systems Inc. have shown the effectiveness of EDC clinical study technologies, having facilitated over 1,000 study initiations, highlighting the increasing dependence on such technologies in research.
Furthermore, advancements such as the Data Quality application in REDCap illustrate the ongoing evolution of EDC systems, aimed at defining and executing quality rules to mitigate discrepancies in project information. Medidata, acknowledged as a pioneer in the EDC clinical study field, has supported over 30,000 trials, showcasing trust and reliability in managing trial data. Besides improving research processes, these medical studies have a significant effect on local economies through job creation, economic growth, and better healthcare outcomes, promoting international collaboration.
For any expert in research involving patients, a comprehensive grasp of EDC clinical study platforms is crucial, as they are key in enhancing the quality and dependability of trial outcomes.
Key Features and Benefits of EDC Systems
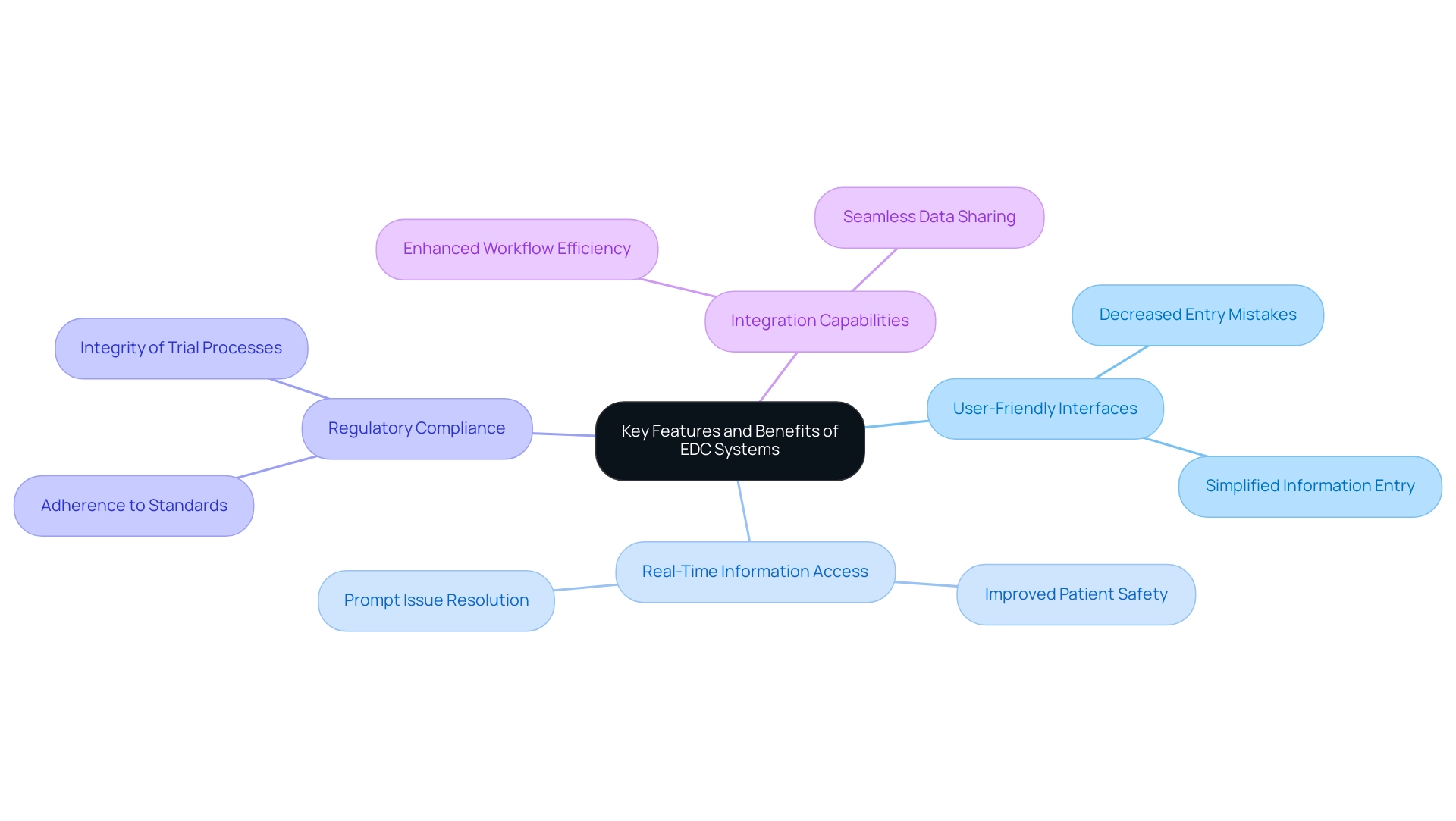
The efficiency of electronic information capture (EDC) platforms in clinical trials is highlighted by several key characteristics that align with comprehensive clinical trial management services:
- User-friendly interfaces: These interfaces simplify information entry processes, significantly diminishing the chances of mistakes that can jeopardize study outcomes.
- Real-time information access: EDC platforms offer immediate insight into trends and issues, facilitating prompt actions that improve patient safety and overall trial integrity. As emphasized by Abu Saleh Mohammad Mosa, Director of Research Informatics at the University of Missouri Institute for Clinical and Translational Science,
In this article, we describe an online EDC and research repository (RDR) platform for CTR, highlighting the significance of such platforms in modern research.
- Regulatory compliance: Built-in functionalities ensure adherence to regulatory standards, including 21 CFR Part 11, which is crucial for maintaining the integrity of trial processes.
- Integration capabilities: The ability to seamlessly connect with other platforms—such as laboratory information management platforms and electronic health records—enhances the overall efficiency of management workflows.
The advantages of using tools in an EDC clinical study are substantial. They result in enhanced information precision, with research indicating that EDC platforms can decrease entry mistakes by as much as 30%. Furthermore, they significantly reduced entry time, with some trials reporting a 40% decrease in time spent on collection compared to traditional methods. Improved patient safety through timely monitoring is another critical advantage. Moreover, these frameworks foster enhanced collaboration among research teams, ultimately contributing to more efficient and effective EDC clinical study processes.
EDC clinical study networks also play a crucial role in enabling feasibility studies and site selection by providing precise information that aids decision-making processes. As shown in a case study titled 'Information Accuracy in Clinical Trials,' information integrity and reliability are crucial to prevent problems during FDA inspections; electronic capture platforms improve information precision, although the accountability for input remains with the users.
The EDC clinical study concluded that EDC systems enhance collection and preparation for statistical processing, saving valuable time compared to traditional paper-based methods, which often encounter delays in structuring and cleaning. Furthermore, the recent launch of the Data Quality application in REDCap assists in defining and implementing quality rules to check for inconsistencies in project information, highlighting advancements in EDC technology. Such developments are anticipated to greatly benefit trials throughout 2024 and beyond, potentially influencing local economies through job creation, economic growth, and healthcare enhancement by improving the overall effectiveness of research.
Essential Criteria for Choosing the Right EDC System
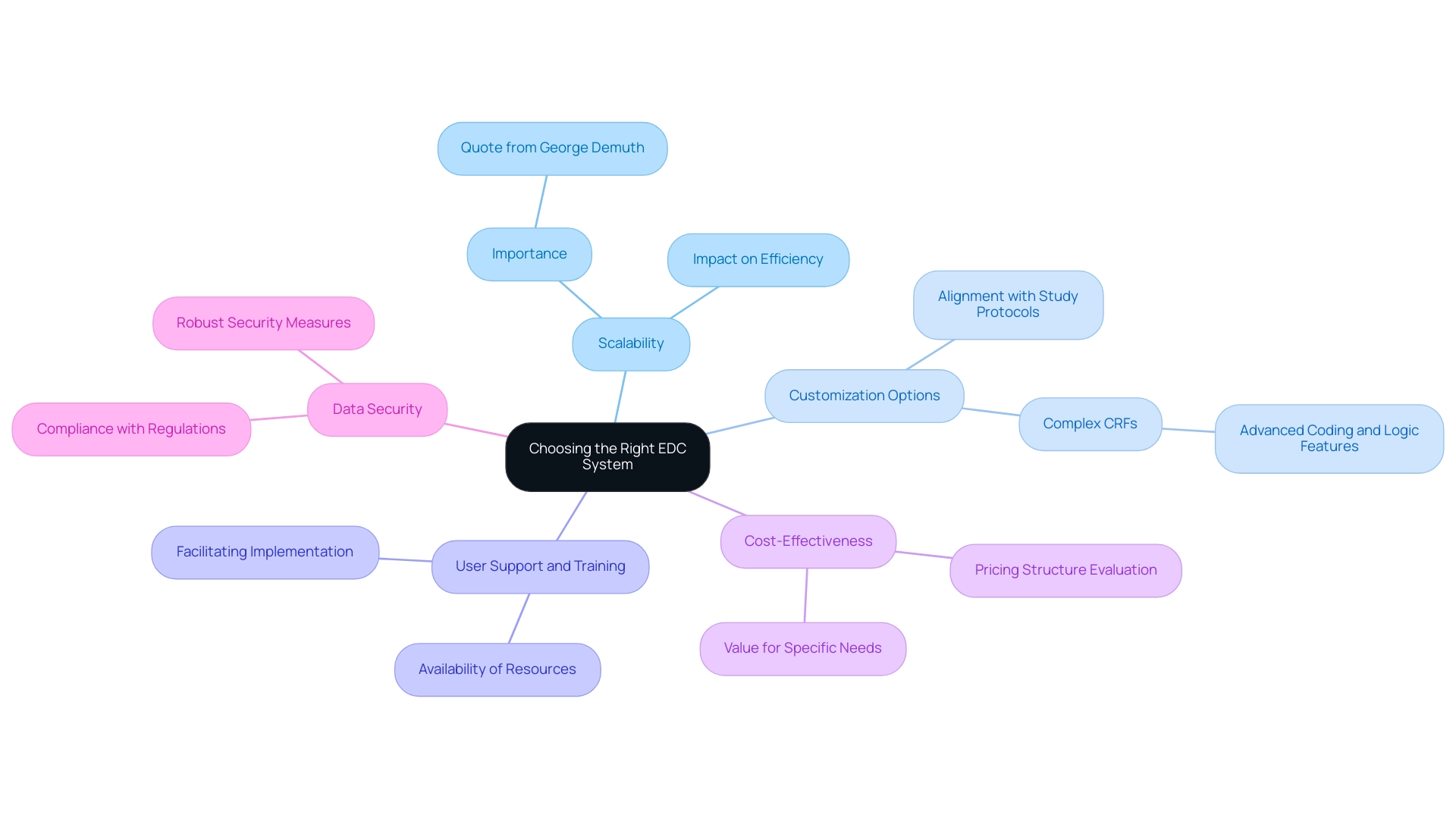
When selecting an electronic data capture (EDC) system for clinical trials, it is crucial to consider several essential criteria:
- Scalability: The chosen system must be capable of accommodating the varying sizes and complexities of your trials. As mentioned by George Demuth, CEO of Stat One, "Scalability is essential to ensure that information management processes can efficiently adjust to the needs of medical research." This aspect can significantly impact the efficiency of data management processes in the EDC clinical study.
- Customization Options: Seek EDC solutions that provide customization to align with specific study protocols and workflows. This flexibility is particularly vital for studies with complex case report forms (CRFs) that may require advanced coding and logic features. A case study titled "Selecting the Right EDC for a Study" highlights that matching study needs with EDC capabilities is crucial for the success of an EDC clinical study, especially for CRFs.
- User Support and Training: Assess the availability of comprehensive customer support and training resources. This support is essential to facilitate your team's implementation and ensure effective ongoing usage of the platform.
- Cost-Effectiveness: Conduct a thorough evaluation of the pricing structure to ensure it fits within your budget while providing the necessary features. A cost analysis can reveal which solutions offer the best value for your specific needs.
- Data Security: It is imperative to confirm that the EDC platform complies with data protection regulations and implements robust security measures to safeguard sensitive patient information.
Based on recent client surveys, EDC solutions have garnered remarkable ratings of 4.98 out of 5 points, demonstrating their effectiveness in research. By diligently evaluating these criteria, you can choose an EDC solution that not only fulfills your research needs but also improves the overall efficiency and results of your EDC clinical study.
Challenges in Implementing EDC Systems
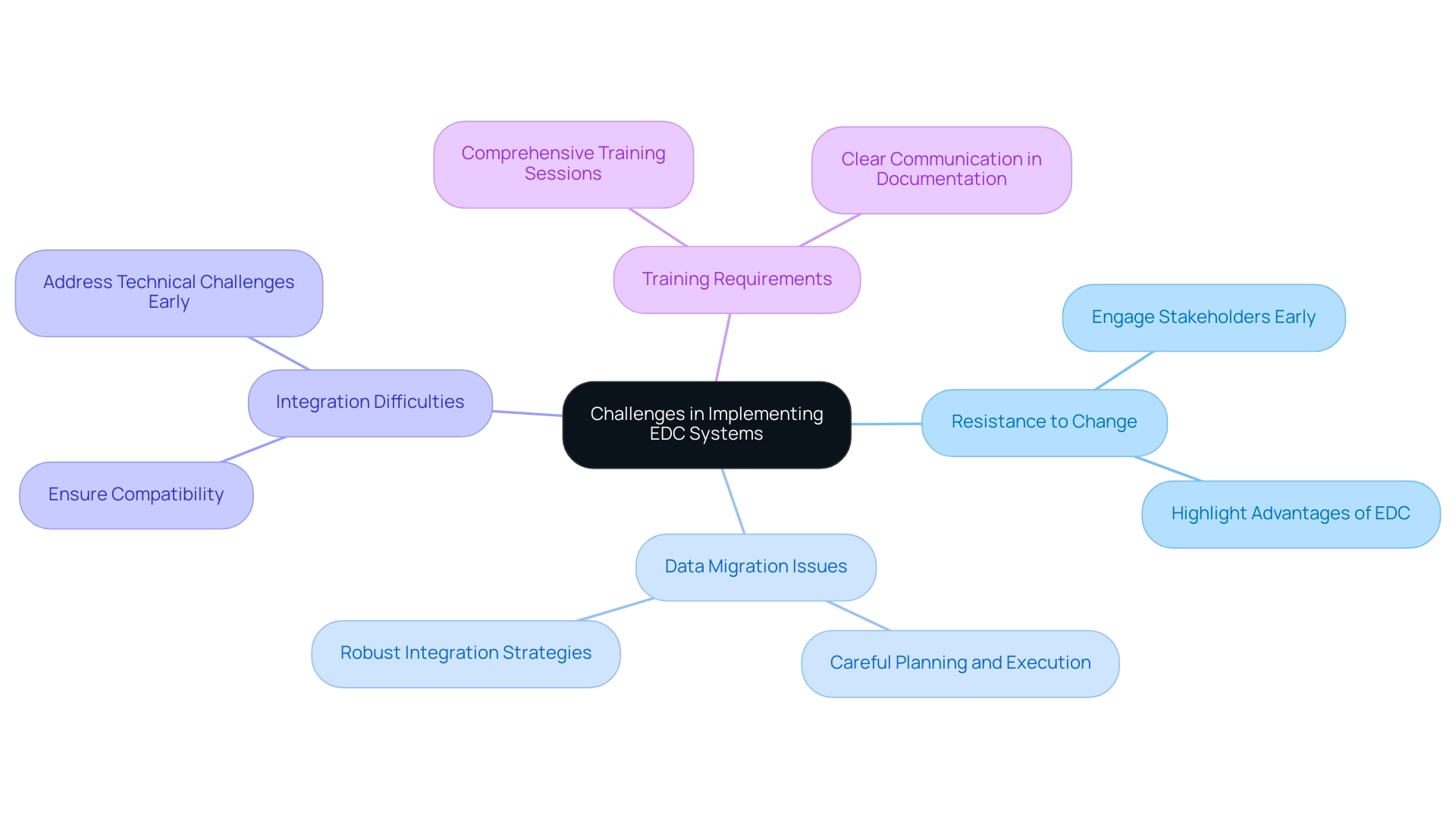
Implementing an electronic information capture (EDC clinical study) framework can present several significant challenges that clinical research teams must navigate effectively. One of the primary hurdles is resistance to change. Team members often feel a strong attachment to traditional data collection methods, which can lead to reluctance in adopting new technologies.
As mentioned by Dimple Rajgor, our objectives are to offer a brief summary of EDC frameworks, their types, and associated advantages and disadvantages, along with a description of commonly utilized EDC platforms and their characteristics. This perspective emphasizes the need for understanding among team members regarding the potential advantages of EDC technologies.
Data migration issues also pose a considerable challenge.
Transferring current information from paper formats or various platforms into a new EDC framework can be intricate and lengthy, necessitating careful planning and execution. Recent reports indicate that data migration issues continue to affect the EDC clinical study, emphasizing the necessity for robust strategies to ensure seamless integration.
Integration difficulties arise when attempting to ensure compatibility with existing frameworks and processes.
Technical challenges can impede the fluid functioning of EDC solutions if not addressed from the outset. Furthermore, training requirements are critical; adequate training for staff is essential for efficient use of the new setup, demanding both time and resources to implement effectively. Notably, the median reading age for information sheets is 15–16 years, underscoring the importance of clear communication in training and documentation to enhance understanding among team members.
To mitigate these challenges, it is essential to engage stakeholders early in the process to build buy-in and support.
Providing comprehensive training sessions can empower staff and enhance their confidence in using the new technology. Developing a clear implementation plan that addresses potential obstacles is vital for success. For instance, the audit of oncology trials managed by the ICR-CTSU highlighted the need for standardized demographic information collection and improvements in the readability of patient materials, which are crucial for the effective implementation of an EDC clinical study.
By proactively tackling these challenges and acknowledging the need for ongoing reassessment and re-evaluation of processes, teams can ensure a smoother transition to EDC frameworks and ultimately improve the quality of medical research.
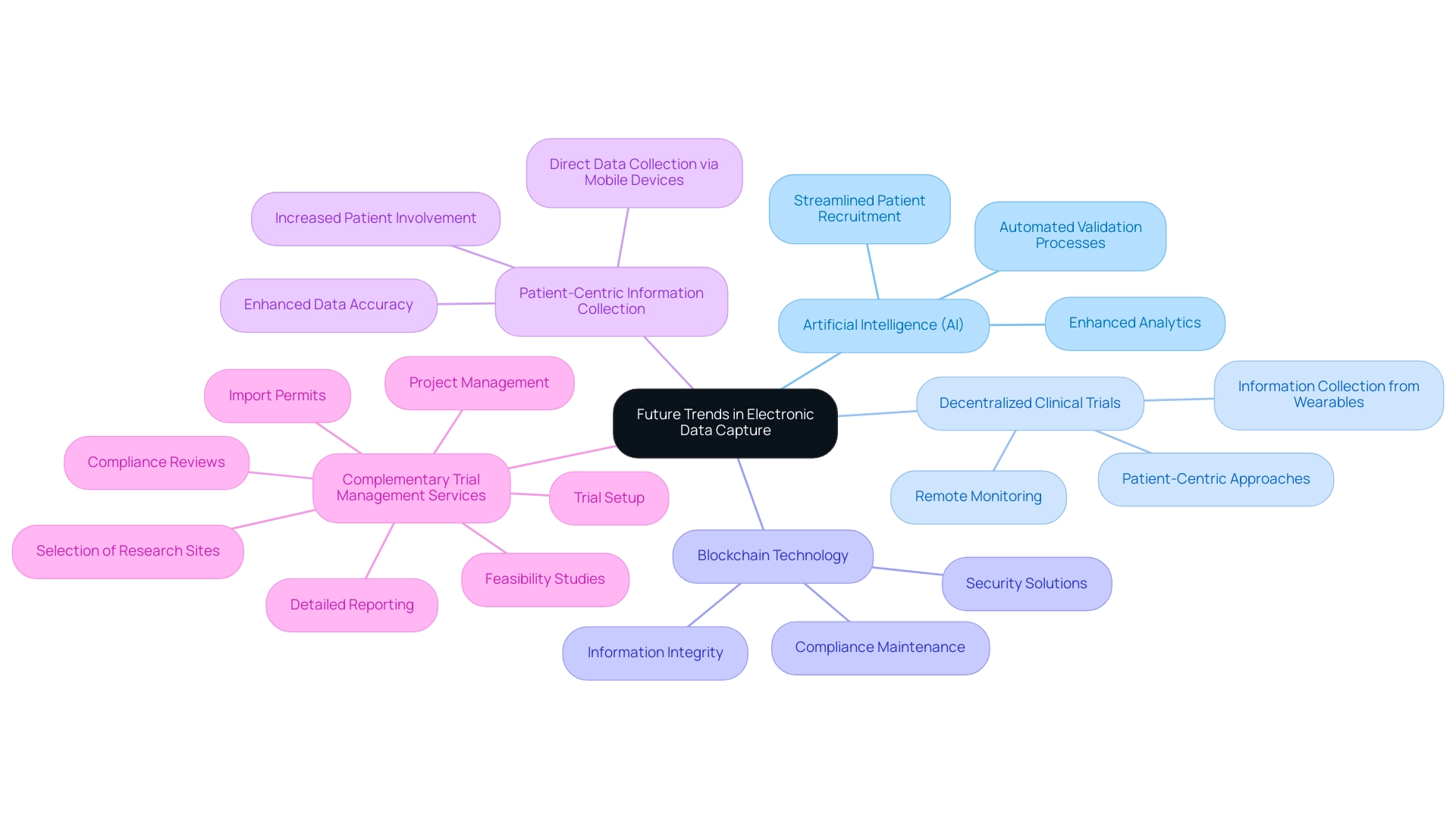
Future Trends in Electronic Data Capture
The landscape of Electronic Information Capture (EDC) is undergoing transformative changes influenced by several key trends that are reshaping its future:
- Artificial Intelligence (AI): The integration of AI technologies into EDC frameworks significantly enhances analytics, streamlines patient recruitment, and automates validation processes. The influence of AI on electronic information capture is profound, enabling more efficient and accurate management in the EDC clinical study.
- Decentralized Clinical Trials: The increase in remote monitoring and decentralized trial approaches has heightened the necessity for EDC platforms capable of gathering information from various sources, including wearables and mobile applications. This shift towards patient-centric approaches facilitates comprehensive information collection, ultimately improving trial outcomes. Significantly, the phase-3 segment maintains the largest market share of 50% in the electronic information capture systems market, highlighting the significance of these innovations.
- Blockchain Technology: The adoption of blockchain for ensuring information integrity and security is gaining momentum, offering a tamper-proof solution for storage. Its potential to safeguard sensitive information is crucial in maintaining the trust and compliance necessary in the EDC clinical study.
- Patient-Centric Information Collection: EDC systems are increasingly prioritizing patient engagement, enabling more direct information collection through mobile devices. This approach not only enhances data accuracy but also fosters greater patient involvement in the research process.
Complementing these advancements, our comprehensive trial management services include:
- Feasibility studies
- Selection of research sites
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Detailed reporting on study status and adverse events
As we navigate these trends, it is essential to apply due diligence and strategic planning, similar to reducing risks in commercial real estate financing, to ensure successful execution of EDC frameworks. As these trends continue to evolve, staying informed will empower researchers to leverage cutting-edge technologies, enhancing data capture efficiency and ultimately improving study outcomes. Furthermore, the North America market dominated the Global Electronic Data Capture Systems Market by Region in 2021 and is projected to reach a market value of $1.2 billion by 2028, highlighting the growing relevance of these systems in the EDC clinical study.
Conclusion
The discussion surrounding Electronic Data Capture (EDC) systems highlights their transformative role in clinical trials, shifting the paradigm from traditional methods to advanced technological solutions. With features such as user-friendly interfaces, real-time data access, and robust regulatory compliance, EDC systems enhance data accuracy and streamline workflows, significantly improving the overall efficiency of clinical research. The ability to integrate with various data sources further underscores their importance in modern clinical trials.
Selecting the right EDC system involves careful consideration of essential criteria, including:
- Scalability
- Customization
- User support
- Cost-effectiveness
- Data security
These factors are pivotal in ensuring that the chosen system aligns with the specific needs of clinical trials, ultimately enhancing data management processes and trial outcomes. However, the implementation of EDC systems does not come without challenges. Resistance to change, data migration issues, and the need for comprehensive training can impede successful adoption. Addressing these challenges proactively is crucial for a smooth transition and the realization of the benefits offered by EDC technologies.
Looking ahead, the future of EDC systems is shaped by emerging trends such as:
- Artificial intelligence
- Decentralized clinical trials
- Blockchain technology
These advancements promise to enhance data collection methods, improve patient engagement, and ensure data integrity, further solidifying the role of EDC systems in advancing clinical research. As the landscape continues to evolve, keeping pace with these innovations will be vital for professionals in the field to leverage the full potential of EDC systems, ultimately leading to improved healthcare outcomes and economic growth.




