Introduction
Ecuador's clinical trial landscape presents a unique opportunity for medical device research, influenced by its diverse demographics and distinct healthcare infrastructure. Navigating this environment requires a comprehensive understanding of local regulations, successful trial histories, and the capabilities of Contract Research Organizations (CROs) that operate within the region.
From the initial stages of selecting a CRO to the complexities of regulatory compliance and patient recruitment, various factors play a pivotal role in the success of clinical trials. By exploring the intricacies of this landscape, stakeholders can better position themselves to leverage Ecuador's potential for innovative medical solutions that cater to a wide array of patient populations.
Understanding the Clinical Trial Landscape in Ecuador
Ecuador's clinical study landscape is shaped by its unique demographics, geographical diversity, and healthcare infrastructure. Understanding these elements is crucial when selecting a Medical Device CRO Ecuador. Start by investigating the categories of medical device studies that have taken place in the country, including significant first-in-human experiments like PAVmed's successful PortIO™ implantations.
Evaluate the success rates and challenges encountered by these studies, as they underscore the urgent need for solution-driven approaches in the region. Acquaint yourself with regional health agencies, like the Agencia Nacional de Regulacion, Control y Vigilancia Sanitaria (ARCSA), and their functions in supervising research studies. Furthermore, take into account the accessibility of research locations and the expertise of local researchers, as these elements can greatly influence the practicality of your studies.
Medical Device CRO Ecuador provides vital services, such as:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Project setup
- Project management
These services are crucial for navigating the intricacies of research. Collaboration opportunities, akin to the partnership between Greenlight Guru and bioaccess™, can also improve the quality and efficiency of research studies in Ecuador, ultimately bridging gaps in innovation and execution.
Navigating the Regulatory Environment for Medical Devices in Ecuador
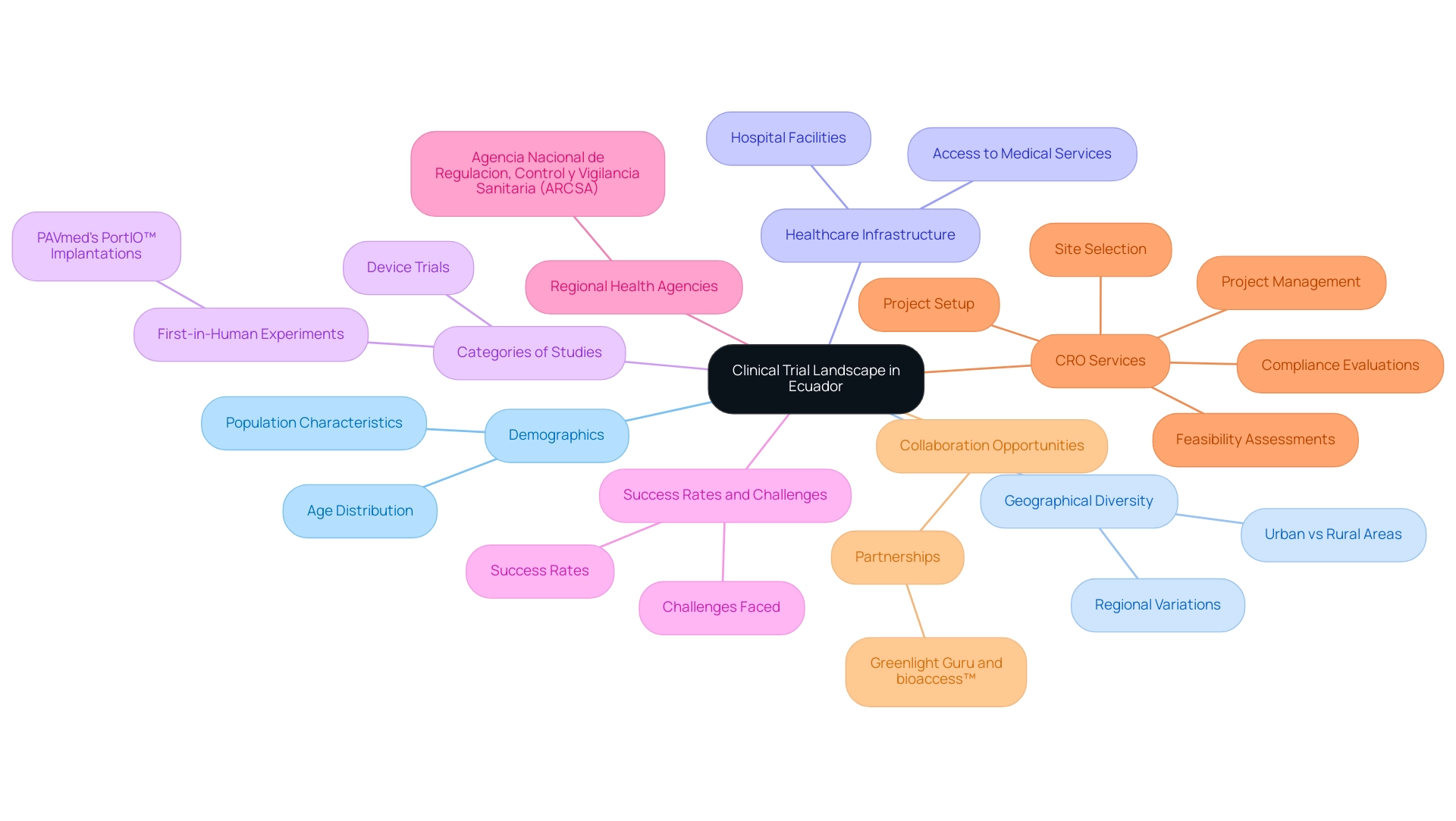
During research studies, it is essential to follow the particular regulatory standards for medical equipment set by the Medical Device CRO Ecuador. Acquaint yourself with the procedures set by ARCSA, including the registration of medical products and the authorization of research studies. Ensure that the Medical Device CRO Ecuador you consider, such as bioaccess®, has a proven track record of compliance with these regulations, as non-compliance can lead to significant delays or even termination of the study.
With expertise from professionals like Katherine Ruiz, a Regulatory Affairs expert, bioaccess® effectively manages documentation and submissions, ensuring successful interactions with regulatory bodies. Their extensive clinical study management services encompass:
- Feasibility assessments to evaluate site viability
- Site selection to determine optimal research locations
- Compliance reviews to guarantee adherence to regulations
- Study setup for efficient initiation
- Import permits for investigational tools
- Detailed reporting on study status and adverse events
These services tackle the common challenges encountered by medical equipment startups, such as regulatory hurdles, competition, recruitment issues, and financial constraints, ensuring a streamlined process for advancing medical trials with the help of a Medical Device CRO Ecuador.
Leveraging Diverse Patient Populations for Effective Trials
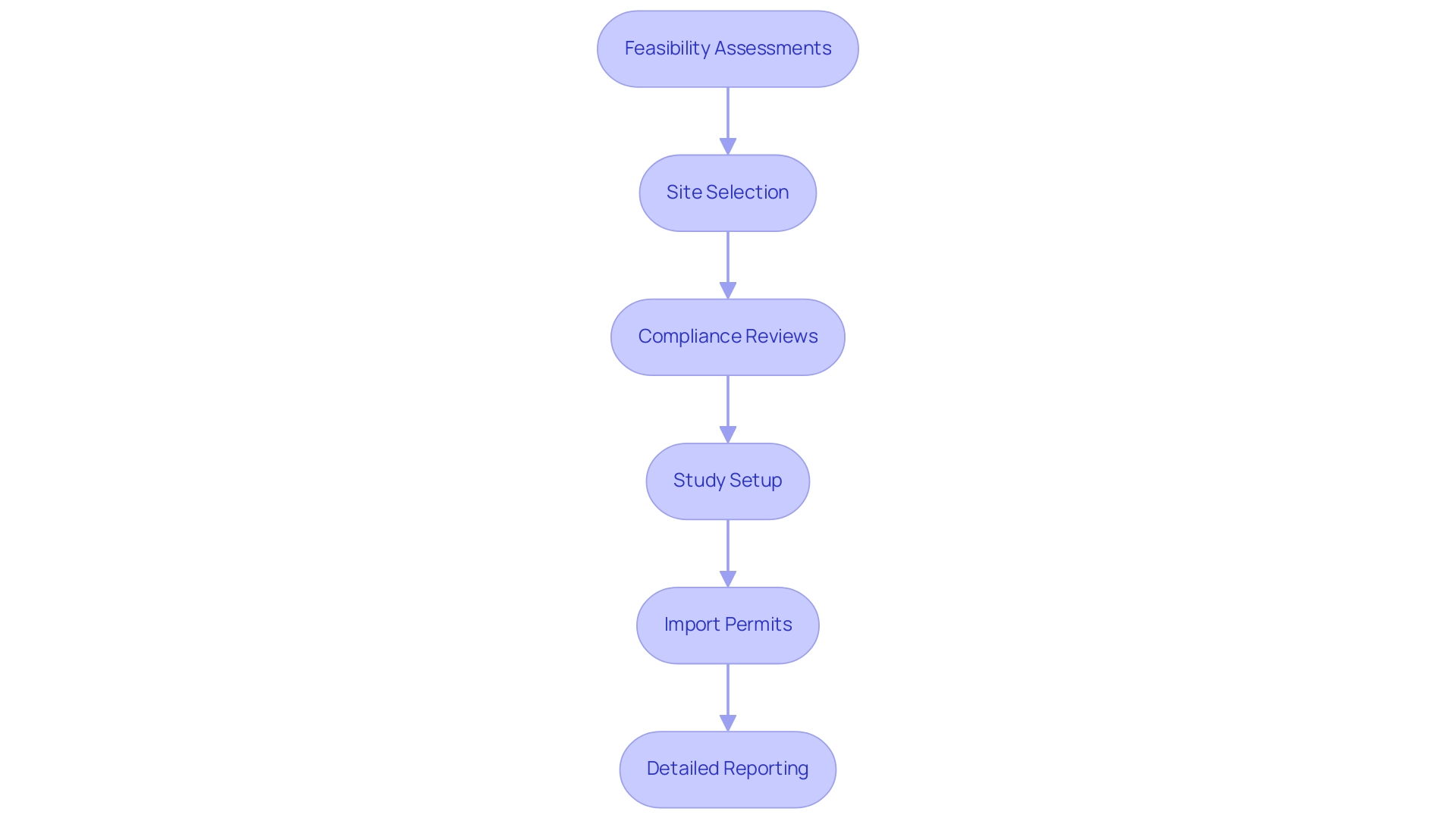
Ecuador, with its rich tapestry of ethnic and socio-economic backgrounds, provides valuable insights into the efficacy and safety of medical instruments, particularly through the efforts of Medical Device CRO Ecuador across different populations. When selecting a CRO, inquire about their strategies for recruiting diverse patient cohorts, as demonstrated by ReGelTec's successful Early Feasibility Study of HYDRAFIL™ for treating chronic low back pain in Colombia, where all eleven patients were successfully treated under remote proctoring via Zoom. A Medical Device CRO Ecuador with established connections in various communities, such as GlobalCare Clinical Trials in partnership with bioaccess™, will be more effective in enrolling participants that reflect the target demographic for your medical device.
Furthermore, GlobalCare Clinical Studies has achieved a reduction in clinical subject recruitment time by over 50% and a retention rate exceeding 95%, showcasing the effectiveness of their approach. Additionally, consider the CRO's experience in managing studies that require cultural sensitivity and awareness, ensuring compliance with local regulations and enhancing recruitment and retention rates.
Evaluating the Expertise of Medical Device CROs in Ecuador
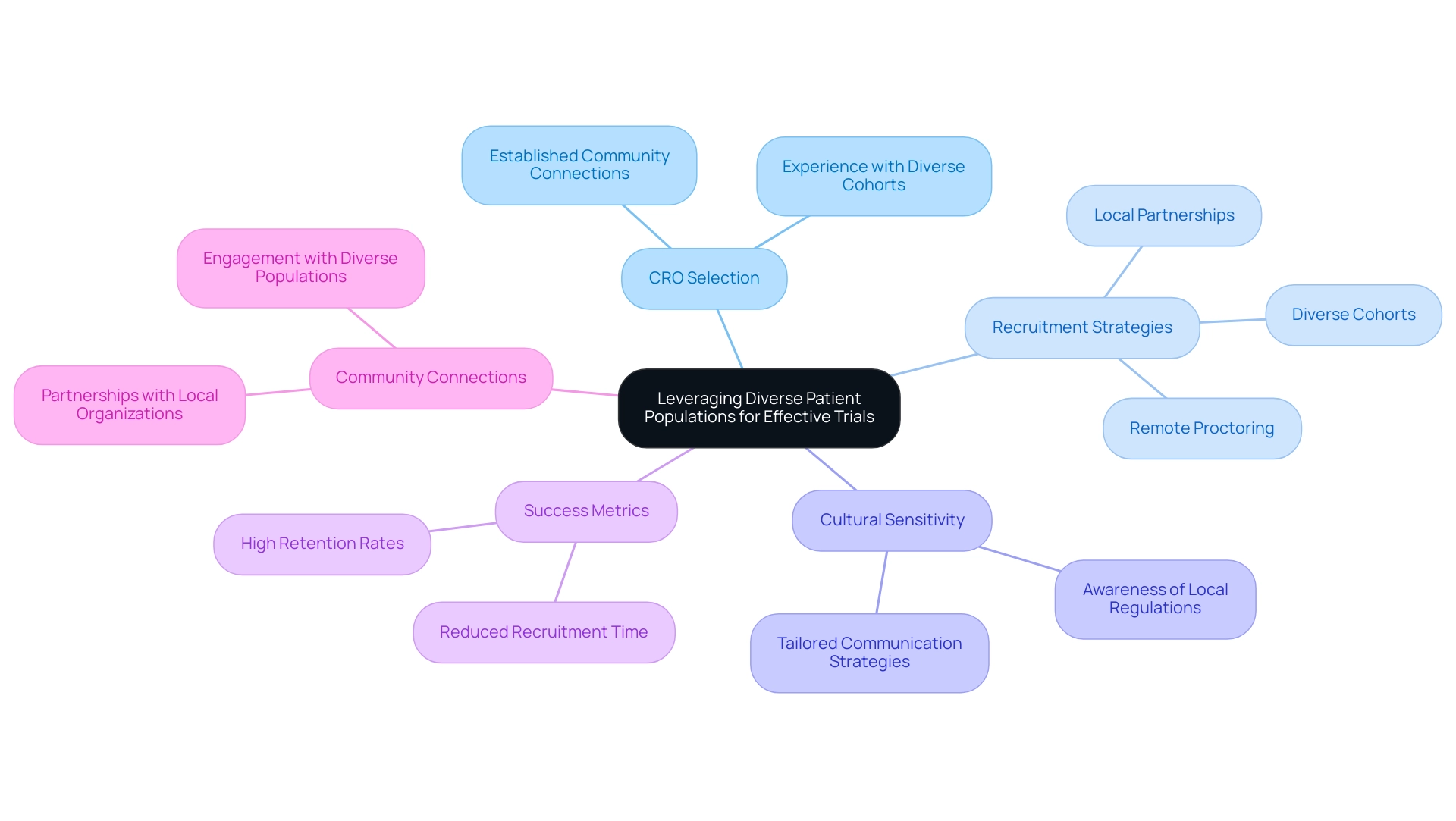
When assessing the Medical Device CRO Ecuador, especially for medical equipment evaluations, consider their vast experience of over 20 years in Medtech and specialized knowledge in executing various kinds of research, such as:
- Early-Feasibility
- First-In-Human
- Pilot
- Pivotal
- Post-Market Follow-Up Research
Evaluate the success rates of these tests and request detailed case studies or references from previous clients to gauge their performance and reliability. It's crucial to evaluate the CRO's team composition, ensuring they include qualified personnel, such as:
- Regulatory Affairs experts like Katherine Ruiz
- Experienced Clinical Trial Managers like Oswaldo Amaya
who have a proven track record in medical device research.
Furthermore, ask about their extensive project management abilities, including:
- Feasibility and selection of research sites
- Compliance reviews
- Timelines
- Resource allocation
- Communication strategies
to ensure they can effectively meet your specific study requirements.
Fostering Effective Collaboration with Your Chosen CRO
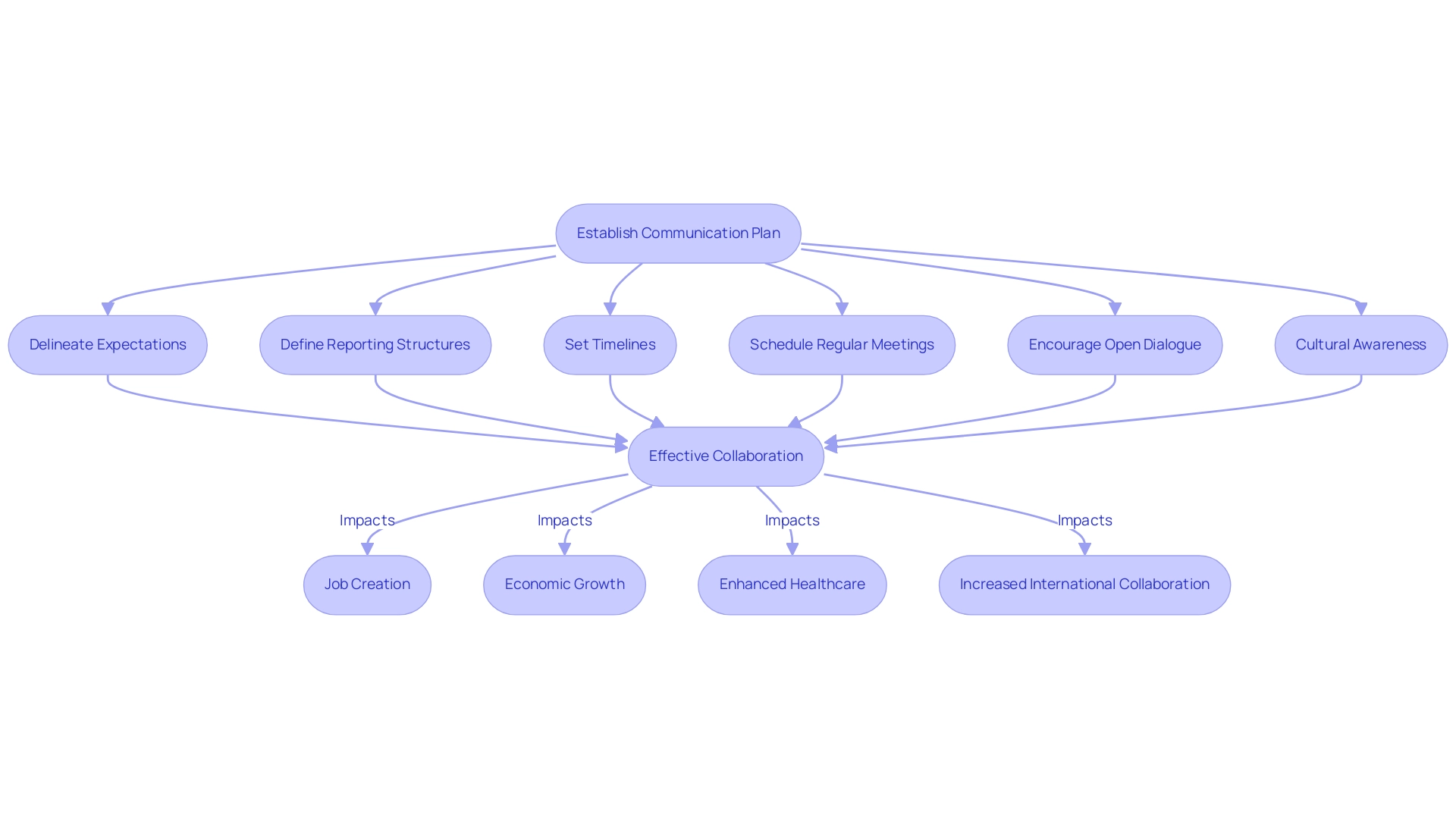
When selecting a Contract Research Organization (CRO), it is essential to establish a comprehensive communication plan that clearly delineates expectations, reporting structures, and timelines. This framework not only acts as the backbone of effective collaboration but also incorporates the comprehensive management services provided by the CRO, including:
- Feasibility assessments
- Site selection
- Compliance reviews
- Setup
- Import permits
- Project management
- Reporting
- Evaluation and feedback on documentation to adhere to country requirements
Regularly scheduled meetings should be implemented to review progress, address challenges, and align objectives effectively.
Creating an environment that emphasizes transparency and open dialogue is crucial; team members from both the sponsor and the CRO should be encouraged to share insights and provide feedback freely. Such proactive communication enhances trial management efficiency and cultivates a robust working relationship, pivotal for achieving successful outcomes. Furthermore, as our Medtech associates participate in research activities, the ripple effect on local economies appears through:
- Job creation
- Economic growth
- Enhanced healthcare
- Increased international collaboration
Ultimately promoting global health advancements.
As highlighted in the case study on cultural awareness in communication, understanding cultural biases is also crucial for effective collaboration in diverse teams. The pandemic has necessitated a reevaluation of traditional employment methods, emphasizing the need for organizations to adapt their communication strategies in the context of global virtual teams (GVTs). Furthermore, it is noteworthy that advanced degree holders earn an average salary 25% higher than those with only a bachelor's degree, reinforcing the value of education in roles that demand effective communication skills.
Dr. Zakaria, Associate Professor at the University of Sharjah, notes that 'the workplace of tomorrow will be filled with cultural-code switchers, people who accommodate diversity, equality, and inclusivity, fostering collaboration without fear.' This perspective underscores the importance of cultural awareness in communication strategies within clinical trials, ultimately paving the way for more effective partnerships. Reporting on serious and non-serious adverse events is a critical aspect of maintaining compliance and ensuring participant safety, further emphasizing the need for a thorough understanding of cultural differences in communication.
Conclusion
Ecuador's clinical trial landscape offers a fertile ground for medical device research, characterized by its diverse demographics and supportive healthcare infrastructure. Success in this environment hinges on the careful selection of a Contract Research Organization (CRO), which plays a crucial role in navigating regulatory complexities, ensuring compliance, and facilitating effective patient recruitment. Understanding local regulations set forth by authorities such as ARCSA is imperative, as is the evaluation of CROs based on their proven track record in managing trials that reflect the unique needs of Ecuador's patient populations.
The importance of leveraging the country’s rich ethnic and socio-economic diversity cannot be overstated. This diversity provides invaluable insights into the performance and safety of medical devices across various demographics. Effective recruitment strategies, as demonstrated by successful partnerships between CROs and local communities, significantly enhance trial efficacy and participant retention. A CRO’s experience with culturally sensitive practices and community engagement is vital for optimizing trial outcomes, ensuring a robust representation of the target population.
Collaboration with the chosen CRO must be underpinned by strong communication strategies that foster transparency and alignment of goals. Regular interactions and feedback loops can greatly enhance the management of clinical trials, ultimately leading to successful results. As the global health landscape continues to evolve, Ecuador stands out as a promising venue for innovative medical solutions, driven by strategic partnerships and a commitment to understanding local dynamics. By tapping into this potential, stakeholders can pave the way for advancements in medical technology that resonate with the needs of diverse patient populations.
Frequently Asked Questions
What factors shape Ecuador's clinical study landscape?
Ecuador's clinical study landscape is influenced by its unique demographics, geographical diversity, and healthcare infrastructure.
What types of medical device studies have been conducted in Ecuador?
Studies in Ecuador include various categories, notably significant first-in-human experiments like PAVmed's successful PortIO™ implantations.
What services does a Medical Device CRO in Ecuador provide?
Medical Device CRO Ecuador offers vital services such as feasibility assessments, site selection, compliance evaluations, project setup, and project management.
Why is regulatory compliance important in Ecuador's medical device studies?
Compliance with regulatory standards set by ARCSA is crucial to avoid delays or termination of studies. Non-compliance can significantly impact the research process.
What role does ARCSA play in medical research studies in Ecuador?
ARCSA supervises research studies and oversees the registration of medical products and the authorization of research activities.
How can a CRO improve patient recruitment for studies in Ecuador?
A CRO with established community connections can effectively recruit diverse patient cohorts, enhancing the representation of the target demographic for medical devices.
What are the common challenges faced by medical equipment startups in Ecuador?
Common challenges include regulatory hurdles, competition, recruitment issues, and financial constraints.
What types of research can a Medical Device CRO in Ecuador conduct?
They can conduct various types of research, including early-feasibility, first-in-human, pilot, pivotal, and post-market follow-up studies.
What qualifications should I look for in a Medical Device CRO team?
Look for a team that includes qualified personnel such as regulatory affairs experts and experienced clinical trial managers with a proven track record in medical device research.
How important is communication when selecting a CRO?
Establishing a comprehensive communication plan is essential for effective collaboration, ensuring clear expectations, reporting structures, and timelines.
What impact does collaboration with a CRO have on local economies?
Collaboration can lead to job creation, economic growth, enhanced healthcare, and increased international collaboration, promoting global health advancements.
Why is cultural awareness important in clinical trials?
Cultural awareness enhances communication strategies within diverse teams, facilitating effective partnerships and ensuring participant safety through proper reporting of adverse events.

