Introduction
As the Medtech sector continues to evolve, Latin America is rapidly establishing itself as a pivotal hub for clinical trials. The region's diverse patient populations, coupled with a growing commitment to healthcare investment, present unique opportunities for Medtech manufacturers seeking to expedite their market entry. Collaborations, such as the one between Greenlight Guru and bioaccess™, are addressing the inherent challenges of clinical research by enhancing regulatory navigation and fostering innovation.
However, the complexities of varying regulations across countries, particularly in emerging markets like Paraguay, require a strategic approach to ensure successful trial execution. This article delves into the emerging landscape of Medtech clinical trials in Latin America, providing insights into conducting research, building collaborative relationships, and overcoming challenges to harness the region's full potential.
The Emerging Landscape of Medtech Clinical Trials in Latin America
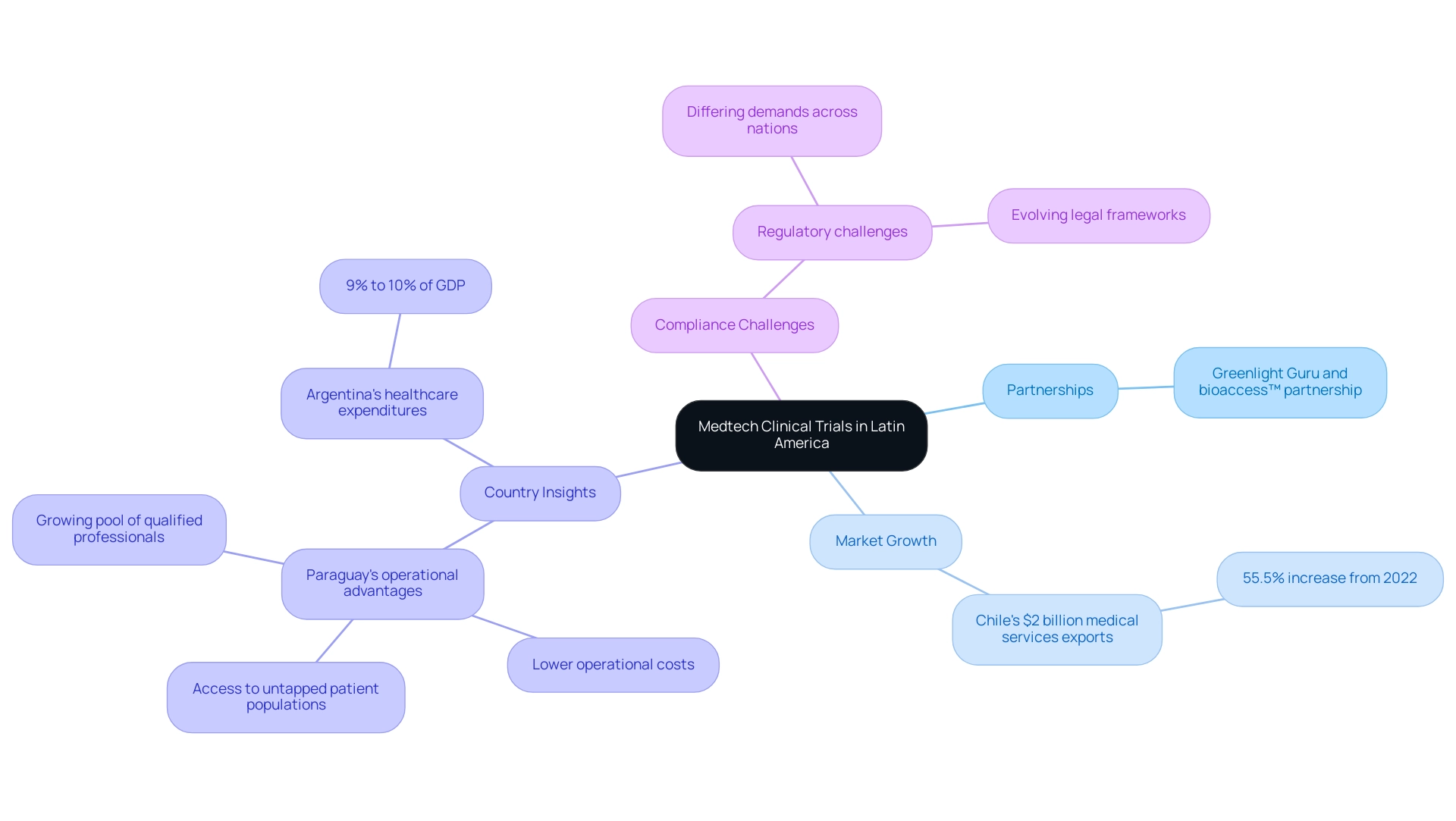
Latin America is becoming an appealing location for clinical research, especially in the Medtech sector, due to the partnership between Greenlight Guru and bioaccess™. This partnership aims to bridge the gaps in clinical research and innovation, enhancing quality of life by helping Medtech manufacturers reach the market faster and with reduced risk. The area's attraction is enhanced by its varied patient groups, changing legal frameworks, and rising healthcare investments. For instance, Chile's medical services exports surged to US$2 billion in 2023, marking a remarkable 55.5% increase from the previous year, which underscores significant advancements in the medical field.
Nonetheless, maneuvering through the compliance landscape presents difficulties, as differing demands across nations complicate the testing process. Countries like Paraguay are emerging as key players, offering favorable conditions such as lower operational costs and access to untapped patient populations, alongside a growing pool of qualified professionals. Efforts to align oversight frameworks across Latin America are improving the region's viability and appeal for clinical studies. Moreover, Argentina's healthcare expenditures, which account for 9% to 10% of GDP, reflect a growing commitment to healthcare development, particularly when compared to higher expenditures in North America and Europe.
Recent policy updates and investment trends emphasize the significance of remaining informed. As Julio G. Martinez-Clark, a leading LATAM Medtech CRO expert, emphasizes, 'Let's continue pushing the boundaries of medical science together!' These advancements are essential for stakeholders planning their research efforts in Latin America, ensuring they take advantage of the region's potential in the Medtech clinical study landscape.
Step-by-Step Guide to Conducting Clinical Research in Paraguay
-
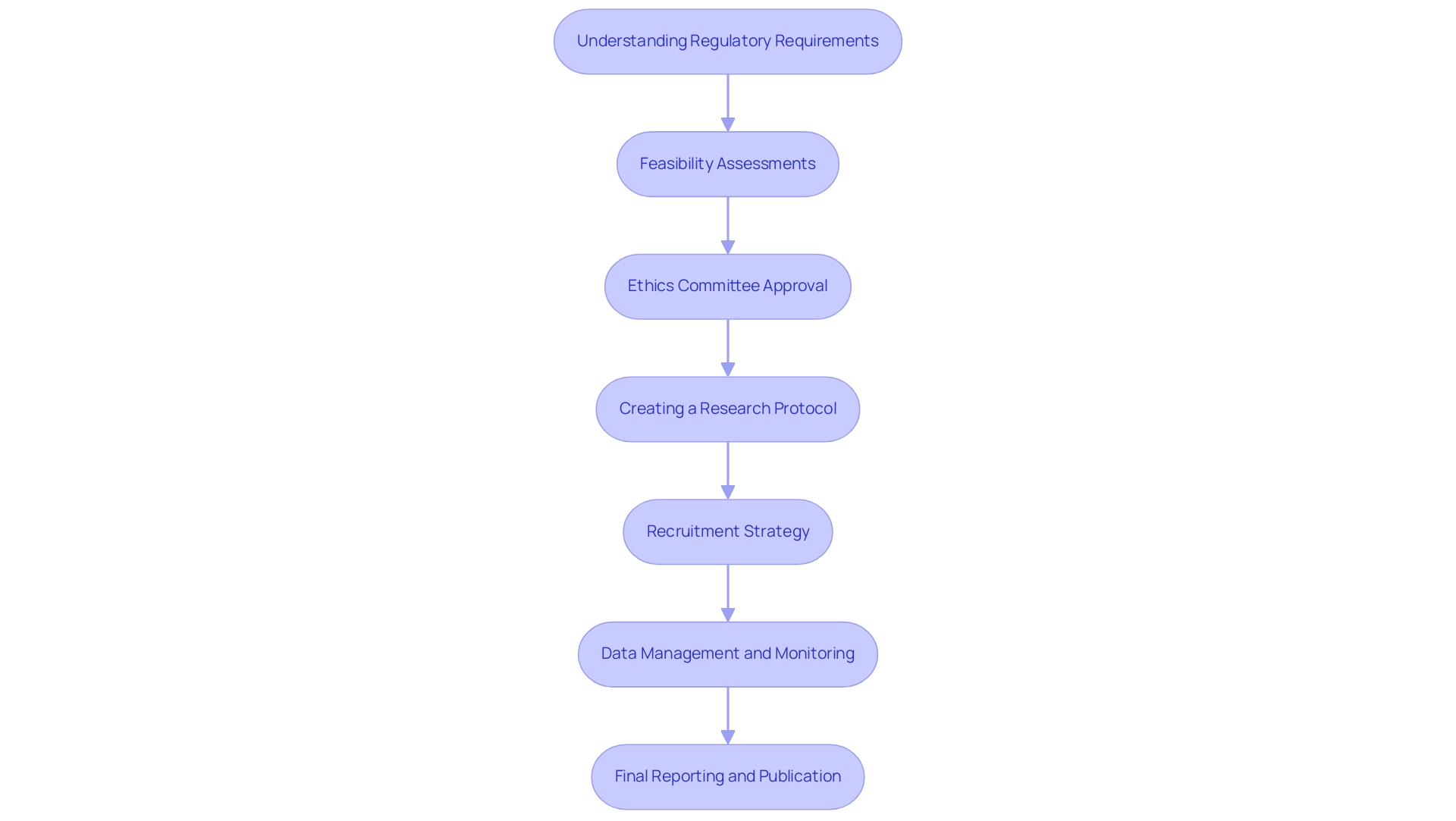
Understanding Regulatory Requirements: Begin by familiarizing yourself with the regulatory landscape in Paraguay and other Latin American countries. The National Agency of Medicine and Food (ANM) supervises clinical studies in Paraguay, necessitating researchers to submit applications that adhere to regional regulations and international standards. Engaging a partner like bioaccess® can provide crucial insights into navigating these regulations effectively, especially as they may differ across countries in the region.
-
Feasibility Assessments: Conduct feasibility evaluations to assess potential locations and patient populations. bioaccess® specializes in this area, leveraging over 20 years of Medtech expertise to evaluate the capabilities and willingness of healthcare providers to participate in clinical trials. For example, earlier successful research in Brazil and Argentina has shown the significance of local partnerships in ensuring effective recruitment and site readiness.
-
Ethics Committee Approval: Secure approval from an ethics committee to ensure the protection of participants’ rights and welfare. Prepare comprehensive research documents, including informed consent forms and protocols, to facilitate this process effectively. Highlighting prior successful approvals can enhance credibility in this area.
-
Creating a Research Protocol: Draft a detailed research protocol that outlines objectives, methodology, and statistical analysis. This document must adhere to both regional regulations and international guidelines, such as Good Clinical Practice (GCP). The group at bioaccess® can help with evaluation and input to guarantee adherence to regional regulations, utilizing their vast experience in comparable research.
-
Recruitment Strategy: Create an effective recruitment strategy to attract suitable participants. Utilize regional media, healthcare providers, and community outreach to engage potential subjects and ensure diverse representation. Successful examples, such as those from previous experiments in Colombia, can offer insights into effective outreach techniques that resonate with local communities.
-
Data Management and Monitoring: Establish a robust data management plan to ensure data integrity and adherence to legal requirements. Implement monitoring processes to oversee trial progress and participant safety throughout the research. With bioaccess®'s project management services, you can streamline these processes, ensuring adherence to timelines and compliance standards.
-
Final Reporting and Publication: Upon completion of the study, compile the results for submission to governing bodies and preparation for publication in peer-reviewed journals. Transparency and adherence to ethical reporting standards are crucial at this stage. Utilizing the knowledge of experts such as Katherine Ruiz, who has substantial experience in affairs related to medical devices, can improve the quality of your submissions, especially in addressing particular nuances in Colombia and beyond.
Building Collaborative Relationships with Local Stakeholders
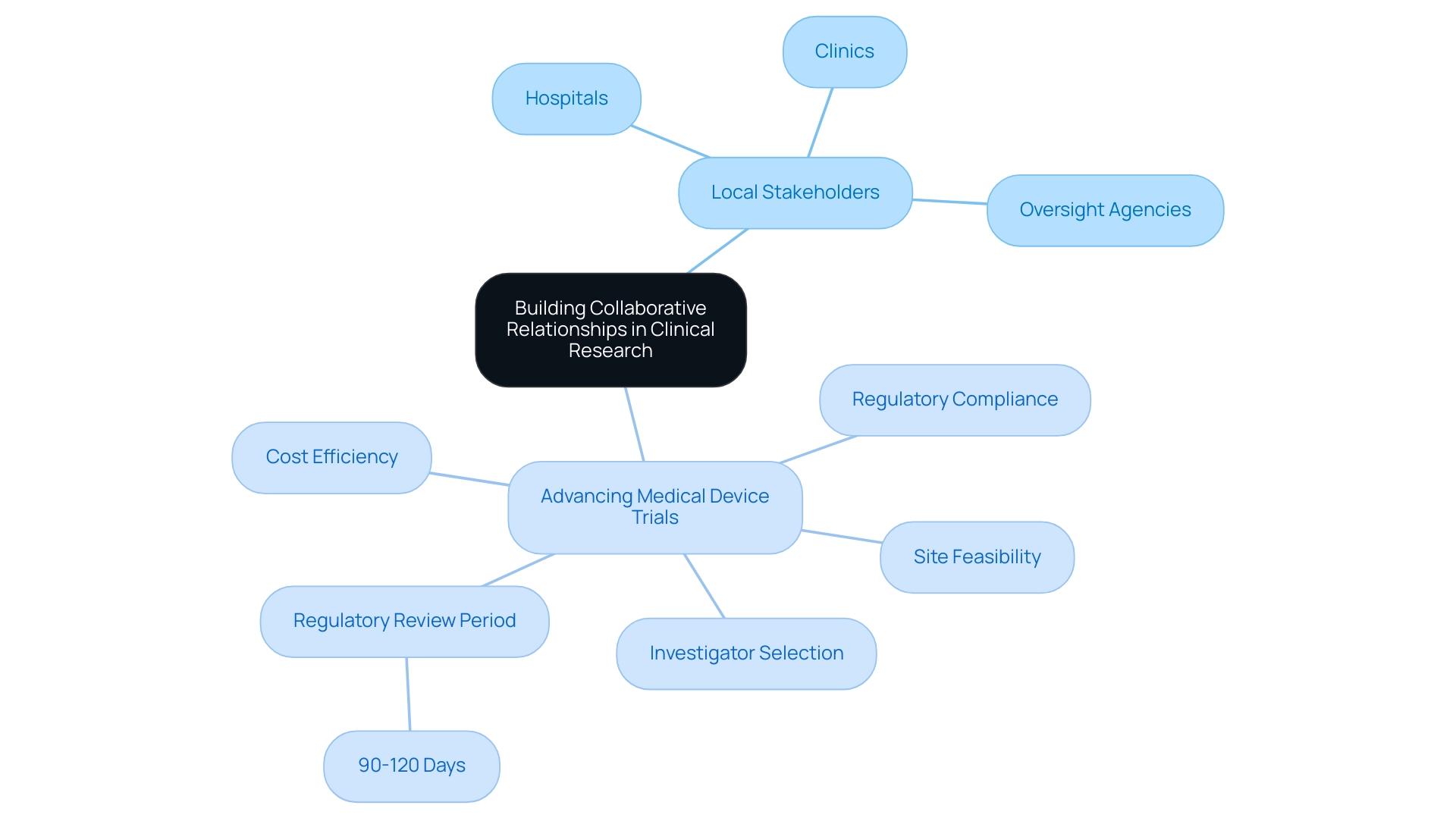
Successful clinical research hinges on building strong relationships with local stakeholders. Start by recognizing key participants in the healthcare ecosystem, including:
- Hospitals
- Clinics
- Oversight agencies
Especially in advantageous locations like Colombia. Engaging with these entities early in the planning process is crucial; it establishes trust and facilitates open communication.
Colombia offers a comprehensive process for advancing medical device trials, encompassing:
- Site feasibility
- Investigator selection
- Regulatory compliance
All while providing the added benefits of cost efficiency and a regulatory review period of just 90-120 days. To foster collaboration, consider hosting informational sessions to familiarize stakeholders with the study objectives and benefits. Regular updates and feedback loops will ensure continued collaboration, enabling a supportive environment for conducting research.
The success of Avinger's OCT-guided atherectomy clinical research in Cali, Colombia, exemplifies the fruitful collaboration with regional experts, demonstrating the high-quality healthcare and robust patient recruitment available in this area. As you navigate this landscape, take proactive steps to engage with these stakeholders, and consider how your project can align with local capabilities and incentives to maximize success.
Navigating Challenges in Clinical Research
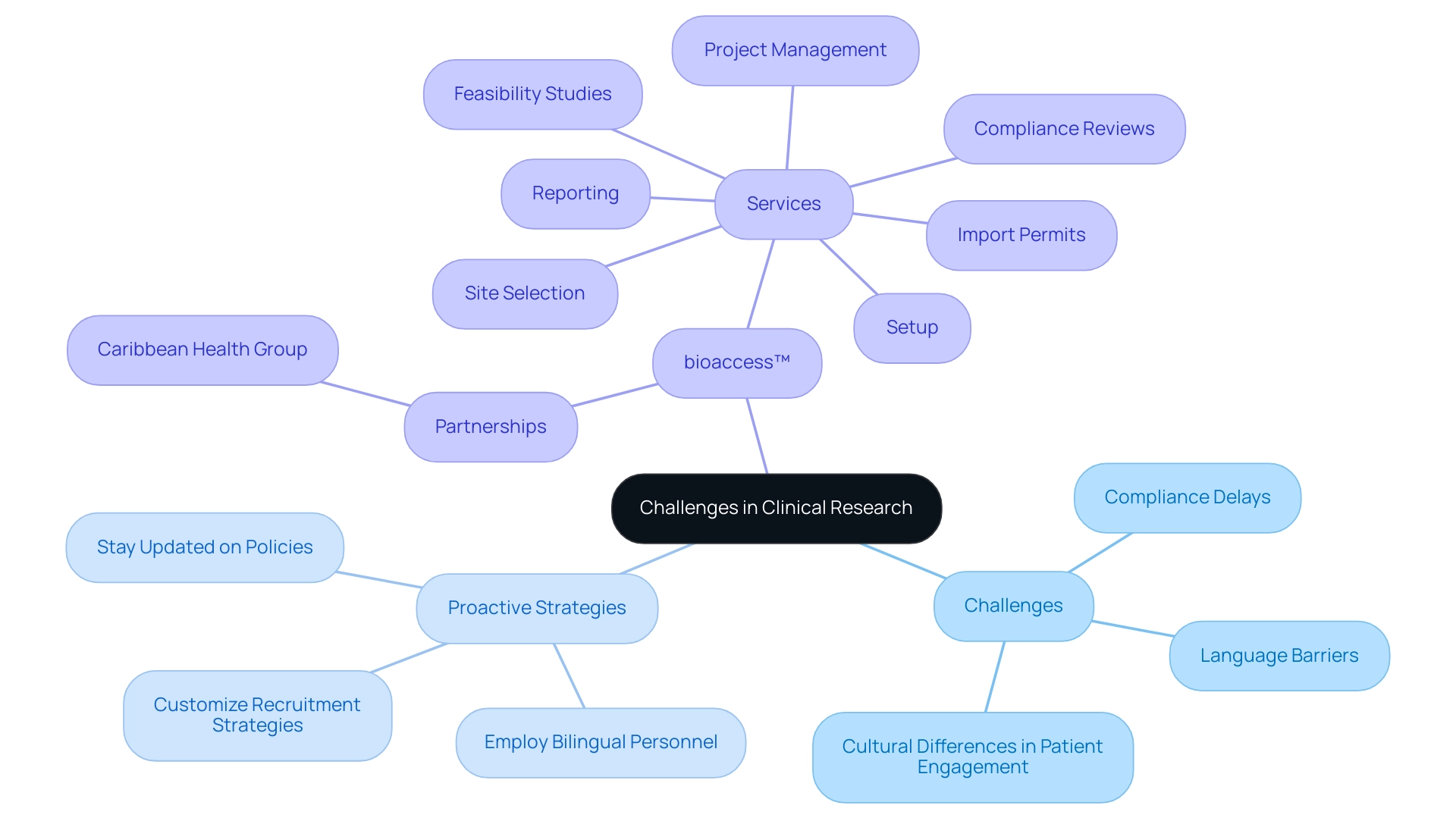
Despite the promising landscape, researchers may encounter various challenges during clinical trials in Paraguay and Colombia. Common issues include:
- Compliance delays
- Language barriers
- Cultural differences in patient engagement
To alleviate these challenges, it is crucial to adopt a proactive method:
- Remain updated on policy changes
- Employ bilingual personnel to aid with communication
- Customize recruitment strategies to connect with nearby communities
For instance, bioaccess™ has successfully navigated regulatory challenges in previous studies by leveraging its extensive experience and local partnerships, which have facilitated smoother approvals and patient recruitment. Furthermore, bioaccess™ partners with organizations like the Caribbean Health Group to position Barranquilla as a leading destination for clinical studies in Latin America. This partnership offers extensive services, including:
- Feasibility studies
- Site selection
- Compliance reviews
- Setup
- Import permits
- Project management
- Reporting
Regularly assessing the progress of your trial and being prepared to adapt your strategies as necessary will ensure participant engagement and compliance with local regulations.
Conclusion
Latin America is poised to become a significant player in the Medtech clinical trial arena, driven by its diverse patient populations and a commitment to enhancing healthcare investments. The strategic collaboration between entities like Greenlight Guru and bioaccess™ exemplifies the potential to overcome regulatory challenges and expedite market access for Medtech manufacturers. The region's evolving regulatory landscape, particularly in countries like Paraguay and Colombia, offers unique opportunities for successful trial execution, provided that researchers adopt a thorough understanding of local requirements and engage effectively with stakeholders.
Successfully navigating the complexities of clinical trials in Latin America requires a multifaceted approach. By conducting feasibility assessments, securing ethics committee approvals, and developing robust study protocols, researchers can lay a solid foundation for their studies. Moreover, fostering collaborative relationships with local healthcare providers and stakeholders is essential to enhance recruitment strategies and ensure participant engagement.
As demonstrated by successful case studies in the region, leveraging local expertise and resources can significantly contribute to the overall success of clinical trials.
In conclusion, the burgeoning landscape of Medtech clinical trials in Latin America presents a wealth of opportunities for innovation and market entry. By staying informed about regulatory changes, addressing potential challenges proactively, and building strong local partnerships, Medtech manufacturers can capitalize on the region's potential. Embracing this approach will not only facilitate successful trial execution but also pave the way for advancements in medical technology that can improve healthcare outcomes across diverse populations.




