Introduction
The clinical research landscape in Panama is rapidly evolving, presenting both challenges and opportunities for researchers aiming to conduct trials in this burgeoning market. With a strategic focus on enhancing the medical device sector, Colombia's initiatives, backed by governmental support, are transforming the region into a hub for clinical studies. As the healthcare infrastructure improves and regulatory frameworks become more accessible, understanding the intricacies of compliance, stakeholder engagement, and patient recruitment is essential for successful trial execution.
This article delves into the critical components of navigating clinical research in Panama, highlighting effective strategies and insights that can lead to impactful outcomes in this dynamic environment.
Understanding the Clinical Research Landscape in Panama
Colombia is swiftly positioning itself as a prime site for research, especially in the medical device sector. The partnership between bioaccess™ and Caribbean Health Group, backed by Colombia's Minister of Health, seeks to turn Barranquilla into a premier location for research studies in Latin America. The nation's strategic geographical location, along with a growing healthcare framework, offers distinctive possibilities for carrying out research studies. As of 2024, Colombia's healthcare system has shown significant improvements, with a reported 30% increase in the number of hospitals and clinics equipped with advanced medical technologies, bolstered by ongoing investments that enhance access to medical services and facilities.
Navigating the regulatory landscape is crucial for researchers. Grasping the guidelines established by the Panamanian Ministry of Health is crucial for compliance and guaranteeing the success of medical studies. Furthermore, cultural factors such as the strong bond between patients and physicians significantly impact patient recruitment and retention. Julio G. Martinez-Clark, CEO of Bioaccess, emphasizes that challenges like drug shortages and restricted awareness of research participation options foster a conducive setting for subject recruitment. He states, 'The absence of research awareness, drug shortages, difficult access to the national public healthcare systems, and the strong bond between patients and physicians make Latin America a fertile ground for subject recruitment and retention.' This context is especially pertinent as Colombia seeks to improve its research landscape.
For example, studies for innovative medical devices, such as cardiac stents and insulin pumps, have successfully recruited participants due to the trust patients place in their healthcare providers and the increasing awareness of the potential benefits of participation. Researchers must become acquainted with the research environment, including local ethics committees and oversight organizations that are essential to the process. Building relationships with these key stakeholders is vital for navigating the regulatory framework effectively and ensuring adherence to all necessary guidelines. Recent studies suggest that understanding of research studies remains low in the region; addressing this gap could significantly improve enrollment rates, thereby enhancing the overall effectiveness of research efforts in Colombia.
Furthermore, GlobalCare Clinical Trials' collaboration with bioaccess™ has accomplished more than a 50% decrease in recruitment time and an impressive 95% retention rate, demonstrating the potential for effective research management services. The focus on extensive services, including feasibility studies, site selection, compliance reviews, and project management, underscores the expertise required to expedite medical device research in Latin America. As the landscape keeps changing, remaining aware of the most recent updates and compliance conditions will be crucial for achieving successful results in studies.
Step-by-Step Process for Conducting Clinical Trials in Panama
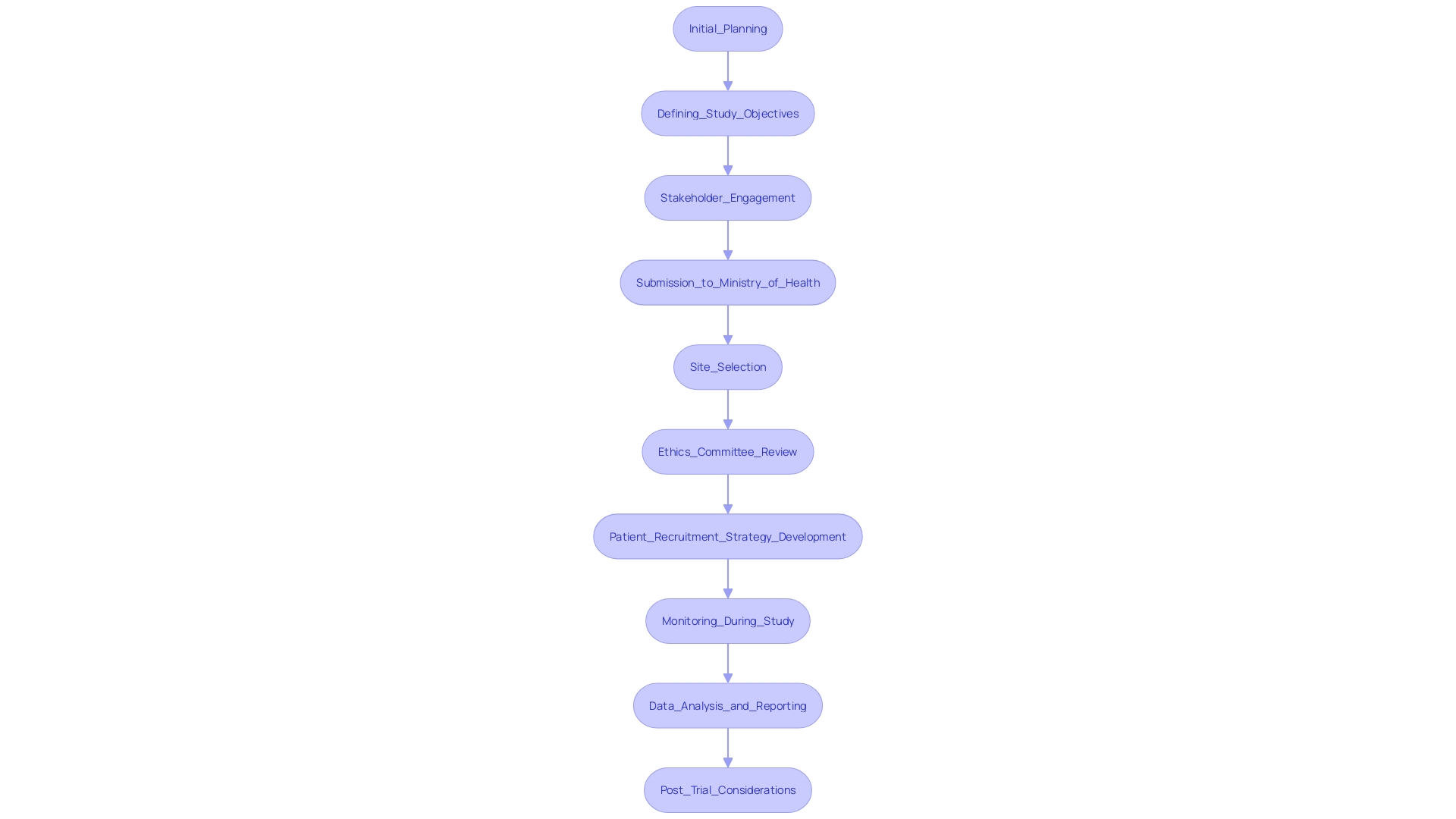
Navigating the regulatory environment for research studies in Panama begins with meticulous initial planning, a hallmark of the comprehensive study management services offered by bioaccess®. Clearly defining the objectives of the clinical study and identifying the target patient population is essential. Engaging stakeholders early in the process is crucial for gathering insights, fostering collaboration, and securing support, significantly enhancing the success of the initiative.
Once objectives are established, the next step is submission to authorities. The research protocol must be prepared and submitted to the Panamanian Ministry of Health for approval. Adhering to local legal requirements is paramount, ensuring that all documentation, such as informed consent forms and safety monitoring plans, meets the necessary standards. Based on a recent announcement from the Ministry of Health, "Maintaining the integrity of medical trials is our highest priority, and we urge researchers to comply with all official guidelines to ensure a seamless approval process."
Following regulatory submission, site selection is critical. This involves identifying and selecting appropriate clinical sites based on various factors, including the site's experience, patient demographics, and geographical reach. Conducting feasibility studies to assess site capabilities is vital, ensuring chosen locations can effectively manage the test. For instance, a recent cardiovascular trial in Panama successfully recruited a diverse patient demographic, highlighting the importance of strategic site selection.
Subsequently, the protocol must be submitted to a local ethics committee for review. Addressing any concerns or recommendations from the committee is essential to ensure adherence to ethical standards, safeguarding the integrity of the research.
In parallel with regulatory and ethical considerations, developing a robust patient recruitment strategy is imperative. This strategy must adhere to ethical guidelines while effectively targeting the appropriate patient population. Statistics indicate that nearly 60% of potential participants drop out during the recruitment process, underscoring the need for effective strategies. Moreover, educating site personnel on the protocol and compliance measures is crucial to ensure high-quality data collection and adherence to study standards.
Once preparations are complete, the clinical study can commence. During this phase, closely monitoring compliance with the protocol is important, implementing regular data collection and safety evaluations throughout the duration to ensure participant safety and data integrity.
Upon conclusion of the test, the next step involves data analysis and reporting. Analyzing the data according to predefined statistical methods will yield meaningful insights. A comprehensive report detailing the findings must be prepared, ensuring transparency and accuracy in interpreting results.
Lastly, post-trial considerations are critical. Preparing for obligations such as follow-up with participants and disseminating results to stakeholders is essential. Addressing any compliance or ethical responsibilities for continued patient care further underscores the commitment to participant welfare and the integrity of the research process. Katherine Ruiz, a specialist in compliance matters for medical devices and in vitro diagnostics in Colombia, spearheads these initiatives with a solid background in securing market approval for innovations, improving the overall success of research studies in the region.
Building Relationships with Local Stakeholders
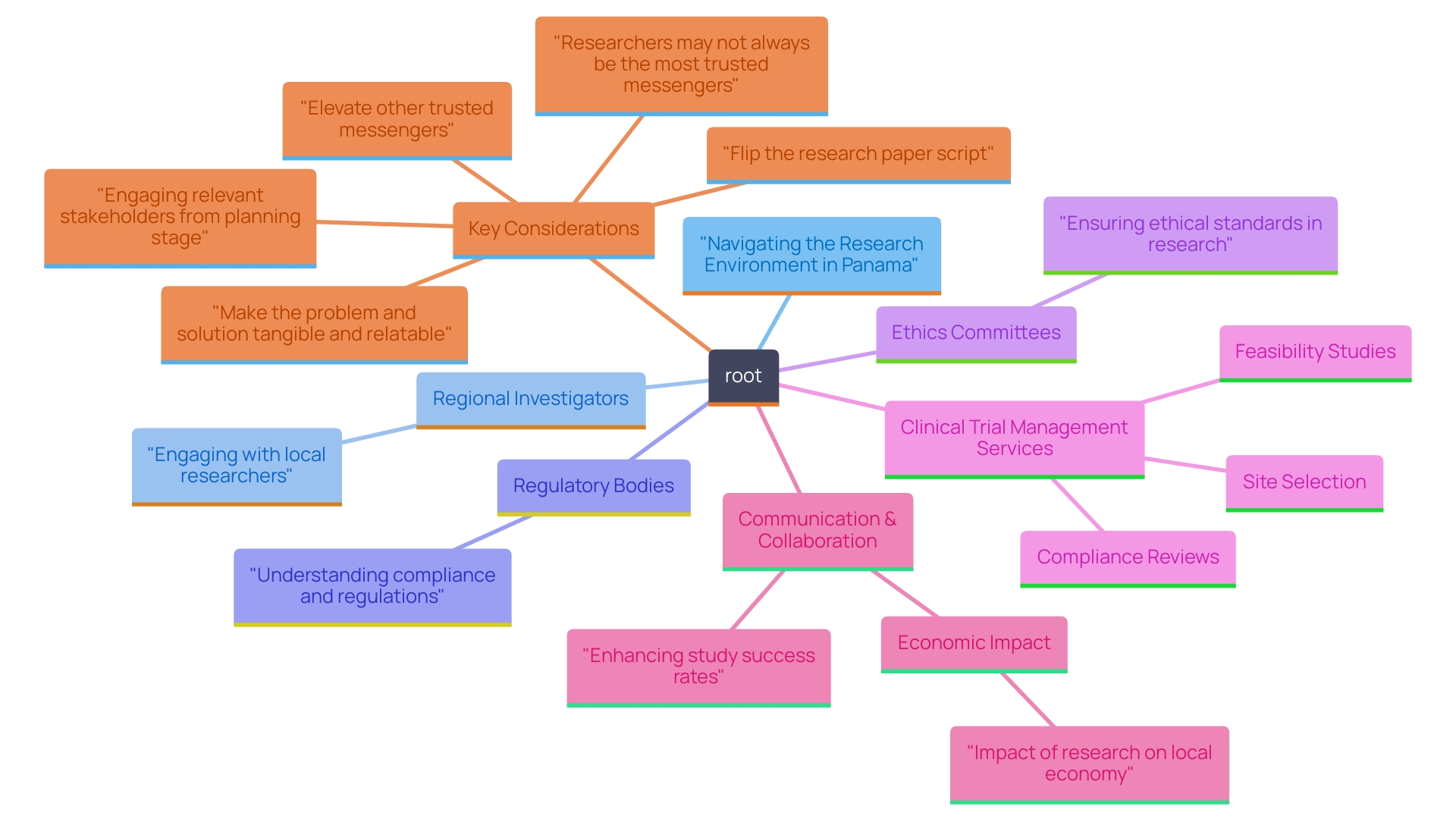
To successfully navigate the research environment in Panama, it is crucial to cultivate strong connections with key stakeholders, including regional investigators, regulatory bodies, and ethics committees. Our comprehensive clinical trial management services encompass:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Reporting
—all crucial elements for successful studies. Start by identifying potential collaborators through participation in regional conferences, such as the Panama Clinical Research Symposium and the Latin American Clinical Trials Conference, which serve as key venues for engagement.
Establishing open communication channels is paramount, as it demonstrates a commitment to ethical practices and transparency. Engaging with local experts not only enhances recruitment efforts but also yields invaluable insights into cultural nuances and patient engagement strategies. A clinician partner aptly noted, 'Coming from [different] disciplines, we speak a different ‘language’ and see things very differently, which is why more team building and communication training would have been helpful.' This highlights the need for ongoing dialogue and collaboration.
According to recent statistics from [insert source or context], effective stakeholder engagement can enhance study success rates by up to 30%, highlighting the significance of these relationships. Regular updates and feedback loops are essential in maintaining strong relationships throughout the testing process. Moreover, our Medtech colleagues and we, during the execution of studies in the regions we operate in, generate waves of effect on the economy, including job creation, economic growth, and healthcare enhancement. Such collaborative efforts not only promote adherence to regional regulations but also significantly improve accessibility and success rates, ultimately benefiting both the research team and regional stakeholders.
Navigating Regulatory Compliance
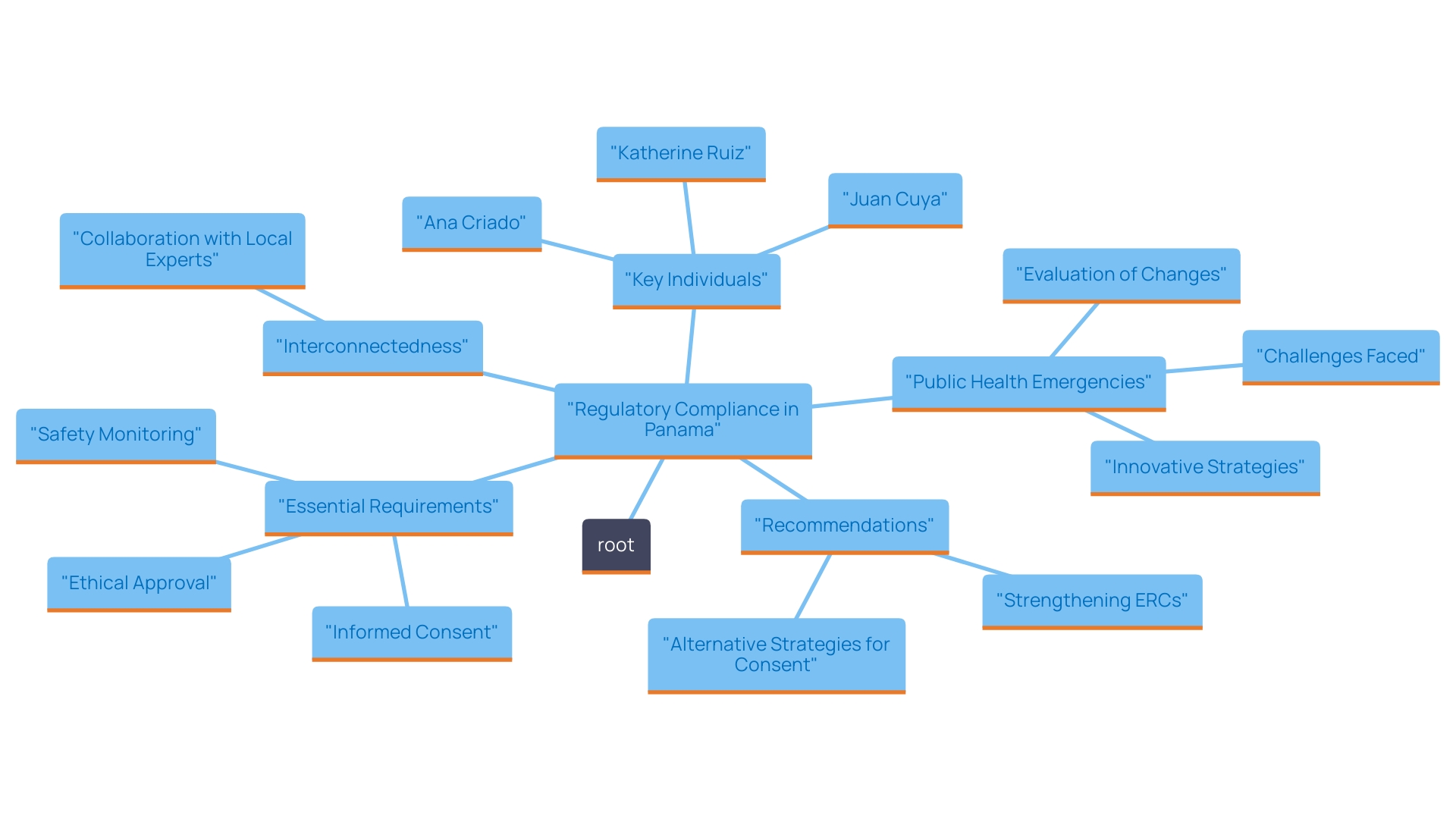
Navigating the regulatory environment in Panama requires a comprehensive understanding of local laws and guidelines, especially for research studies. Researchers must ensure that all study protocols align with the regulations set forth by the Panamanian Ministry of Health, which encompass essential requirements such as:
- Ethical approval
- Informed consent
- Robust safety monitoring
Recent changes in regulations emphasize that as of 2024, compliance statistics indicate a 15% increase in successful approvals for clinical studies, reflecting an evolving landscape. Given these developments, it is crucial to stay updated on any recent changes in regulations and guidelines, as these can significantly impact both ongoing and future studies.
Collaboration with local legal and compliance experts is invaluable in this context. Ana Criado, our Director of Regulatory Affairs, brings extensive expertise in biomedical engineering and health economics, ensuring compliance and effective trial management. Katherine Ruiz, a specialist in medical device and in vitro diagnostic guidelines, offers essential insights for navigating intricate compliance environments. Additionally, Juan Cuya, MD, as a Clinical Trial Associate, offers a strong understanding of compliance matters and project management, further supporting researchers in achieving successful outcomes.
As one legal expert pointed out, 'Grasping the subtleties of regional regulations is essential for the success of trials in Panama.' This highlights the importance of strategic partnerships with our experts, which can effectively minimize the risk of delays or complications during the research process. By leveraging local expertise, researchers can facilitate a smoother pathway for conducting clinical studies in an environment where regulatory compliance is paramount.
Conclusion
The clinical research landscape in Panama presents a unique blend of challenges and opportunities that researchers must navigate to achieve successful trial outcomes. As highlighted, a strong focus on regulatory compliance, stakeholder engagement, and patient recruitment is essential. The improvements in Colombia’s healthcare infrastructure and the strategic initiatives aimed at enhancing the medical device sector signify a promising direction for clinical trials in the region.
Understanding the regulatory framework set by the Panamanian Ministry of Health is crucial for ensuring adherence to guidelines and successful trial execution. The emphasis on building relationships with local stakeholders, including investigators and ethics committees, cannot be overstated. These collaborations foster trust and facilitate effective communication, ultimately enhancing recruitment efforts and improving overall trial success rates.
As the clinical research environment in Panama continues to evolve, staying informed about regulatory changes and leveraging local expertise will be vital. Researchers who strategically approach compliance and stakeholder engagement will be better positioned to navigate the complexities of clinical trials, leading to impactful outcomes that benefit both the medical community and the patients they serve. The potential for growth in Panama's clinical research sector is substantial, and with the right strategies in place, it can become a significant hub for innovative medical research in Latin America.




