Introduction
In the dynamic landscape of medical device development, early feasibility studies serve as a cornerstone for success, particularly in Latin America. These studies not only provide critical insights into a device's functionality and market potential but also align development efforts with the unique healthcare challenges faced in the region. With examples such as ReGelTec's and Avantec Vascular's innovative trials, the importance of these preliminary assessments becomes evident.
They not only help in understanding local needs but also foster essential collaborations with healthcare professionals and regulatory bodies. As the medical device sector continues to evolve, navigating the complex regulatory environments in countries like Argentina and Colombia is paramount for ensuring compliance and enhancing the efficacy of clinical trials. This article delves into the significance of early feasibility studies, the intricacies of regulatory frameworks, and the importance of stakeholder engagement in driving innovation and improving patient care across Latin America.
The Importance of Early Feasibility Studies in Latin America
Initial viability assessments are crucial for medical equipment creators as they offer early insights into the product's functionality, usability, and possible market acceptance. In Latin America, these analyses, such as ReGelTec's Early Feasibility Assessment on HYDRAFIL™ for chronic low back pain and Avantec Vascular's first-in-human clinical trial for an innovative vascular instrument, play a pivotal role in identifying specific healthcare challenges that the instrument aims to address, ensuring that development aligns with local needs.
Additionally, Flow-FX's first-in-human clinical study of its Flow-Screw device for intraosseous antibiotic delivery illustrates the growing opportunities for innovative medical devices in Colombia. Carrying out these studies also cultivates connections with local healthcare experts and stakeholders, which can be invaluable for future clinical research.
bioaccess™ offers extensive clinical trial management services, including:
- Feasibility assessments
- Site selection
- Compliance reviews
These services are essential for navigating the complexities of the Colombian healthcare landscape. By grasping the regional context and navigating the compliance landscape overseen by INVIMA, developers can customize their products to better suit the unique healthcare environment, ultimately enhancing the chances of successful market entry and adoption. The results of these investigations not only advanced medical technology but also contribute to enhancing patient care in the region.
Navigating Argentina's Regulatory Landscape for Medical Device Trials
Carrying out medical instrument assessments in Argentina requires a comprehensive comprehension of the legal structure overseen by the National Administration of Drugs, Food, and Medical Technology (ANMAT). In 2021, there were 52 experiments listed on ClinicalTrials.gov, with only 16 active recruiting projects, emphasizing the necessity for a strong oversight framework to support clinical research. The initial step involves classifying the medical device according to its risk level, which dictates the specific regulatory pathway for approval. A thoroughly prepared Clinical Trial Application (CTA) is vital in this process, encompassing detailed research protocols, informed consent forms, and comprehensive data management plans.
Engaging with local ethics committees early is essential to address all ethical considerations and ensure the integrity of the research. Ongoing communication with ANMAT throughout the evaluation is strongly advised to accommodate any essential adjustments to the protocol. As stated by Researcher B, the usual approval duration for clinical studies spans from 6 to 8 weeks, stressing the necessity of prompt and careful preparation.
Practical instances, like the obstacles recognized in Paraguay, underscore the importance of strong governance frameworks and sufficient training for Ethics Review Board (ERB) members and researchers. The study identified several barriers, including the need for enhanced governance frameworks, which also resonate within Argentina's context. Addressing these issues is vital for breaking the cycle of insufficient scientific production and fulfilling the health needs of the population. In Argentina, clear policies and budget distribution for both profitable and academic research are essential for promoting scientific efforts.
As of 2024, revisions to the ANMAT oversight framework are anticipated to further simplify the approval process for medical devices, which will be vital for improving the effectiveness of clinical studies. By following these compliance standards and maintaining proactive communication with ANMAT, researchers can ensure adherence, enhance the efficiency of their studies, and contribute to the scientific community's progress. In this context, bioaccess® stands out with its comprehensive clinical study management services, including:
- Feasibility studies
- Site selection
- Compliance reviews
- Study setup
- Import permits
- Project management
- Reporting
For example, bioaccess® has successfully navigated intricate compliance challenges in over 50 medical apparatus clinical trials throughout Latin America, showcasing its ability to handle various governance environments effectively. With Katherine Ruiz’s expertise in compliance matters for medical devices and in vitro diagnostics, bioaccess® is well-positioned to navigate the complexities of the legal landscape in Argentina and beyond, driving innovation and ensuring successful clinical outcomes.
Identifying Stakeholders and Building Collaborations
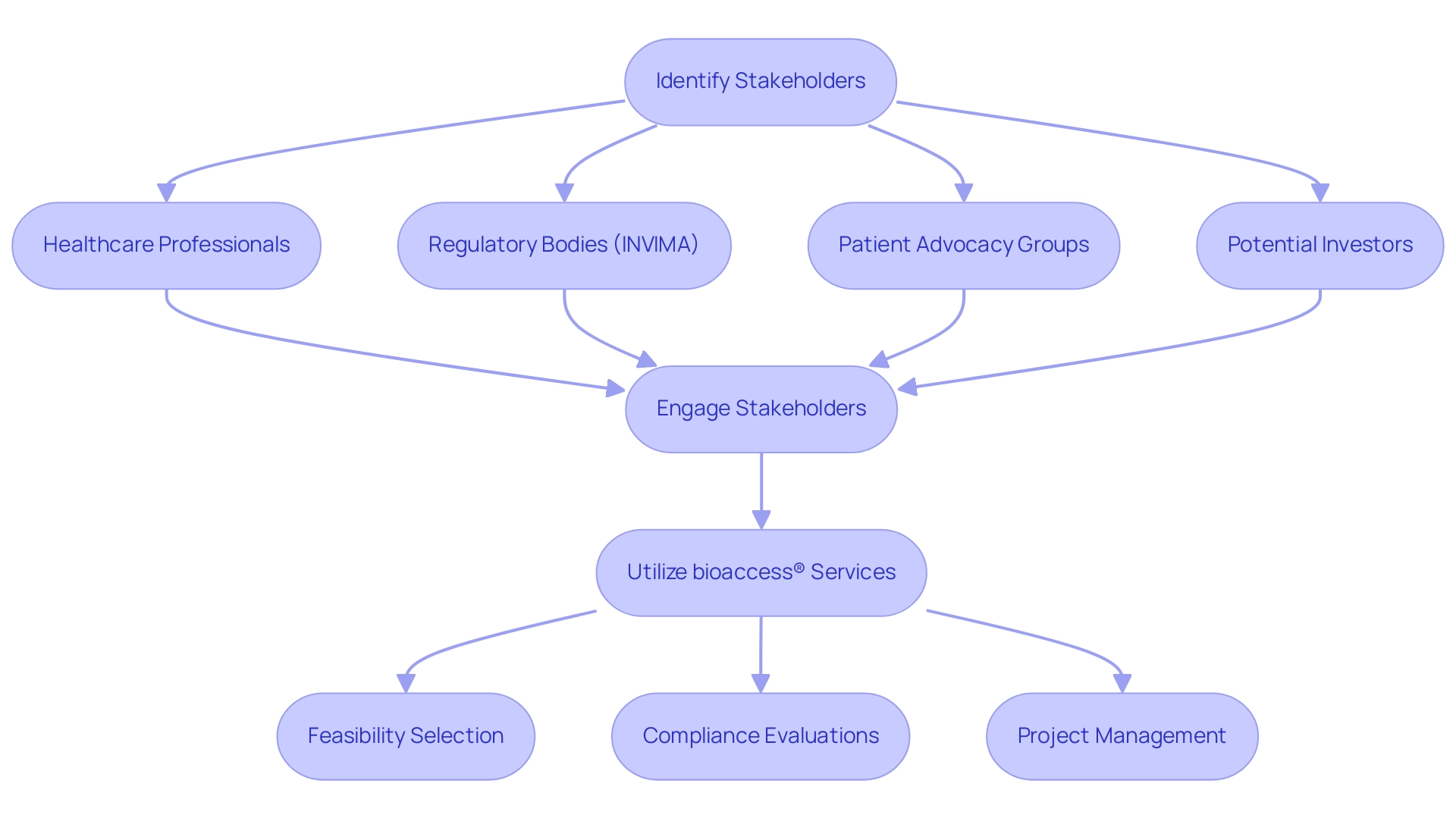
Identifying and engaging stakeholders is a crucial step in conducting early feasibility studies, especially within the legal framework of Colombia, where INVIMA plays a pivotal role. Key stakeholders may include:
- Healthcare professionals
- Regulatory bodies like INVIMA
- Patient advocacy groups
- Potential investors
Start by mapping out the landscape of stakeholders relevant to your medical device and reach out to them to establish relationships. Utilizing the expertise of bioaccess® can assist in navigating these complexities, considering their specialized knowledge in managing Early-Feasibility and First-In-Human research.
bioaccess® provides services including:
- Feasibility and selection of research locations
- Compliance evaluations
- Study setup
- Import permits
- Project management
- Reporting
Consider hosting informational meetings to discuss your research and gather feedback. Collaborating with local healthcare institutions not only provides access to patient populations but also enhances recruitment efforts. Establishing these relationships not only assists in the feasibility assessment but also sets the foundation for future clinical trials and product launches, ensuring compliance with Colombian regulations.
Katherine Ruiz, a compliance affairs specialist with extensive experience at INVIMA, can offer invaluable insights into the oversight landscape, further supporting your project's success.
Designing the Feasibility Study Protocol
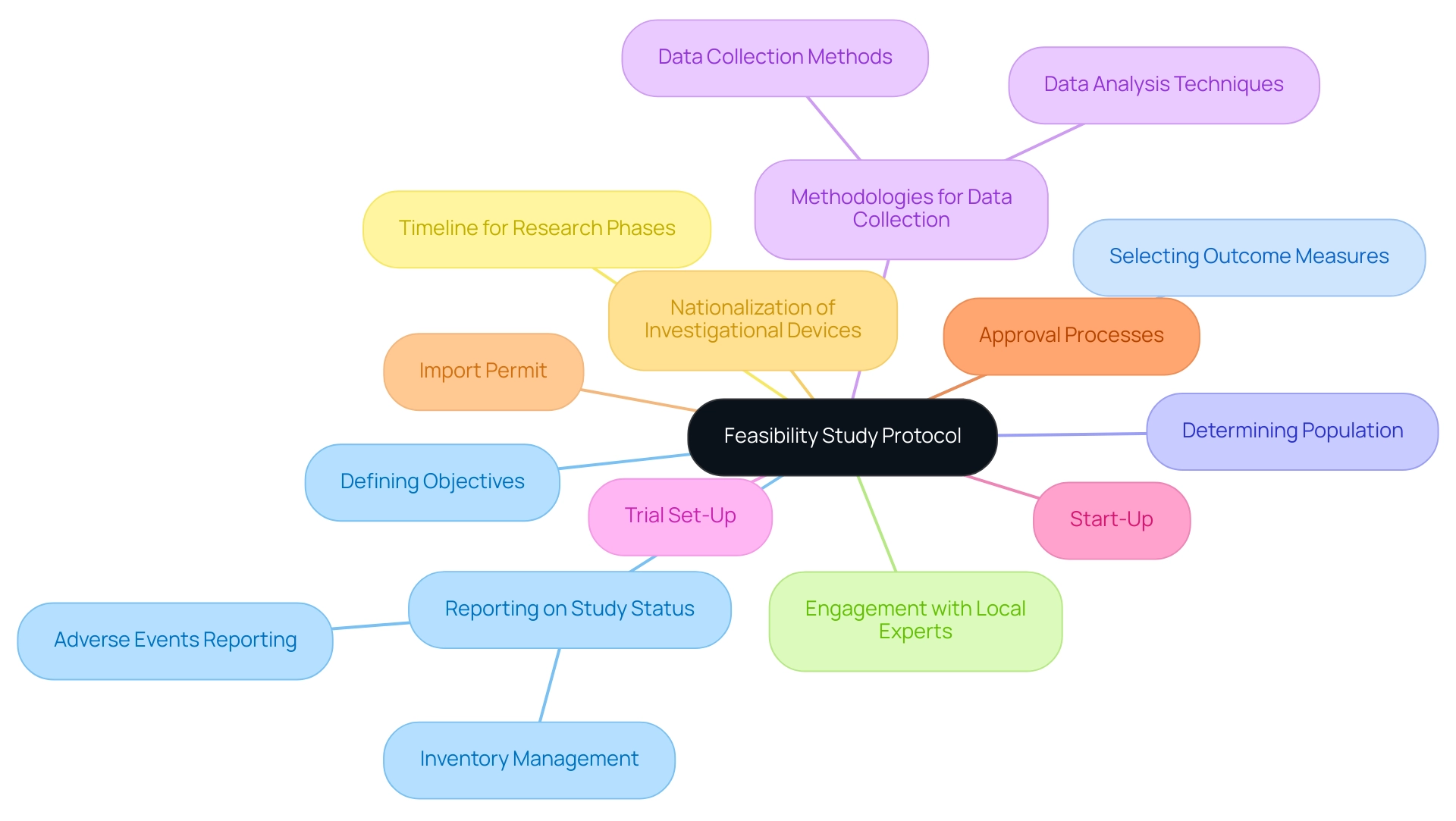
Creating a feasibility research protocol involves several key components, including:
- Defining the objectives
- Selecting appropriate outcome measures
- Determining the population
It is essential to clearly outline the methodologies for data collection and analysis, ensuring they align with regulatory requirements set forth by authorities like INVIMA, the Colombia National Food and Drug Surveillance Institute.
Furthermore, the protocol should include a timeline for each phase of the research to aid in managing expectations and resources effectively. Engaging with local experts during the protocol development phase can provide invaluable insights and enhance the feasibility of the study.
Additionally, the protocol should address critical aspects such as:
- Trial set-up
- Start-up
- Approval processes
- Import permit
- Nationalization of investigational devices
This organized method not only directs the research process but also acts as an essential document for compliance submissions and stakeholder communications, ultimately ensuring adherence to local and international standards. Reporting on study status, including inventory and adverse events, is also crucial for maintaining transparency and regulatory adherence.
Conclusion
Early feasibility studies are critical for the successful development of medical devices in Latin America, offering valuable insights into functionality and market viability while addressing local healthcare challenges. The experiences of companies like ReGelTec and Avantec Vascular highlight the importance of these assessments in building collaborations with healthcare professionals and regulatory bodies.
Navigating the regulatory environments in Argentina and Colombia is essential for ensuring compliance and enhancing clinical trial efficiency. A well-prepared clinical trial application that meets the requirements of ANMAT and INVIMA is crucial, as is early engagement with ethics committees to maintain trial integrity. Upcoming updates to ANMAT's regulatory framework are expected to streamline the approval process, fostering a more innovative landscape.
Engaging key stakeholders, including healthcare professionals and patient advocacy groups, is also vital for improving recruitment efforts and ensuring adherence to regulations. Leveraging specialized clinical trial management services can help developers navigate these complexities effectively.
In summary, early feasibility studies form a foundational element in the development and market entry of medical devices in Latin America. By prioritizing regulatory compliance, stakeholder engagement, and a structured approach to study design, developers can enhance patient care and advance healthcare solutions tailored to the region's unique needs.




