Introduction
In the rapidly evolving landscape of medical technology, early feasibility studies play a pivotal role in assessing the viability of innovative devices before they enter full-scale clinical trials. These preliminary investigations not only gather critical data on safety and effectiveness but also navigate the unique regulatory environments prevalent in regions like Chile and Latin America. By identifying potential challenges early in the research process, these studies allow developers to refine their designs and methodologies, ultimately enhancing the chances of success.
Furthermore, strategic partnerships, such as those between Greenlight Guru and bioaccess™, illustrate the power of collaboration in accelerating Medtech innovations. As US Medtech companies encounter regulatory hurdles and market complexities, understanding the intricacies of conducting feasibility studies becomes essential for driving advancements in patient care and achieving favorable outcomes.
Understanding Early Feasibility Studies for Medical Devices
Initial feasibility analyses for medical devices are preliminary investigations designed to assess the viability of a new device before full-scale clinical trials. They aim to gather initial data on safety and effectiveness, which is crucial for informed decision-making. In Chile and other regions of Latin America, these investigations are especially significant because of the distinct oversight conditions and market demands. They help identify potential challenges early in the research process, allowing for adjustments in design or methodology to enhance the likelihood of success.
Notably, collaborations like that of Greenlight Guru and bioaccess™ exemplify how partnerships can accelerate Medtech innovations and clinical trials, as seen in PAVmed's first-in-human study and ReGelTec's successful treatment of chronic low back pain with HYDRAFIL™.
However, US Medtech companies often encounter significant compliance challenges, language barriers, and fragmented resources that complicate these efforts. Addressing these challenges is essential for effective communication with regulatory bodies and stakeholders, ensuring alignment with local requirements and expectations. This is further demonstrated by Flow-FX's pioneering clinical trial of the Flow-Screw system for intraosseous antibiotic delivery in Colombia, which navigates these complexities while aiming to improve patient outcomes.
Step-by-Step Process for Conducting Feasibility Studies in Chile
- Define Objectives and Scope: Clearly outline the goals of the feasibility assessment, including specific questions you aim to answer regarding the medical instrument. This will guide the entire research process.
- Conduct a Literature Review: Review existing research and data on similar devices to understand market needs, safety concerns, and effectiveness. This background information will guide your research design.
- Develop a Study Protocol: Create a detailed protocol that includes methodology, participant selection criteria, data collection methods, and analytical approaches. Ensure adherence to Chilean regulations and ethical standards, utilizing the expertise of bioaccess® in compliance matters.
-
Obtain Regulatory Approval: Submit your research protocol to the appropriate regulatory bodies in Chile, such as the Instituto de Salud Publica (ISP), for approval. Bioaccess® provides essential support in navigating this process, ensuring that you meet all legal requirements.
-
Recruit Participants: Identify and enlist an appropriate group of participants for the research. Consider factors such as demographics, medical history, and willingness to participate.
-
Conduct the Research: Implement the research according to the approved protocol, ensuring adherence to ethical guidelines and data integrity. Bioaccess® offers comprehensive project management services to ensure smooth execution, demonstrating flexibility and specialized knowledge throughout the trial.
-
Analyze Data: After data collection, examine the results to determine the feasibility of the medical instrument. Look for trends and insights that can guide future development.
-
Report Findings: Prepare a comprehensive report detailing the research's methodology, results, and recommendations. Distribute this to stakeholders, including oversight organizations and potential investors, to promote further advancement of the product.
By adhering to these steps, researchers can effectively carry out initial feasibility studies for medical products in Chile, ensuring they are well-prepared for later stages of research and development. With the assistance of specialists such as Katherine Ruiz, maneuvering through the compliance environment becomes easier, increasing the chances of success in medical equipment trials. Additionally, the extensive service capabilities of the organization, including trial setup, compliance reviews, and project management, are crucial for achieving successful outcomes.
Regulatory Considerations for Medical Device Studies in Chile
In Chile, carrying out feasibility assessments for medical products necessitates compliance with various frameworks, and collaborating with a partner can enhance this process efficiently. Key regulations include:
-
Decree 3/2010: This decree outlines the requirements for the registration of medical devices and the responsibilities of manufacturers and sponsors. Grasping this decree is crucial for guaranteeing adherence and can be supported through expertise in compliance matters.
-
Feasibility and Selection of Research Locations and Principal Investigators (PI): The organization provides essential assistance in pinpointing appropriate research locations and choosing competent principal investigators, guaranteeing that the research is carried out under ideal circumstances and complies with regulatory standards.
-
Ethics Committee Approval: All research must receive approval from an ethics committee, which evaluates the ethical implications of the investigation, particularly concerning participant safety and informed consent. The system assists in managing this process to ensure a smooth approval pathway.
-
Good Clinical Practice (GCP): Familiarize yourself with GCP guidelines, which ensure that research is conducted ethically and that data is credible. Compliance with GCP is mandatory for all clinical research in Chile, and bioaccess® provides comprehensive reviews to ensure adherence to these standards.
-
Reporting Adverse Events: Researchers must have protocols in place for reporting any adverse events that occur during the research. This is crucial for maintaining participant safety and adherence to regulations. bioaccess® provides project management services that encompass monitoring and reporting of both serious and non-serious adverse events to maintain these standards.
By grasping these compliance considerations and utilizing the extensive clinical trial management services offered by bioaccess®, researchers can more effectively navigate the intricacies of carrying out feasibility studies in Chile, reducing risks and improving the credibility of their research.
Best Practices for Conducting Feasibility Studies
-
Engage Stakeholders Early: Involve key stakeholders, including governing bodies, clinicians, and potential users, in the planning phase to gather valuable insights and foster collaboration. This is particularly crucial in regions where regulatory frameworks, such as those established by INVIMA in Colombia, govern clinical trials.
-
Pilot Testing: Conduct pilot tests of the protocol to identify potential issues and refine methodologies before full-scale implementation. This can save time and resources, ensuring compliance with the rigorous standards set by authorities like INVIMA.
-
Continuous Monitoring: Implement a system for ongoing observation of progress and participant safety. Regular check-ins can help identify issues early and ensure adherence to protocols, particularly in the context of Early-Feasibility Studies (EFS) and First-In-Human Studies (FIH) managed by experienced teams like those at bioaccess®.
-
Documentation: Maintain thorough documentation throughout the research process, including all communications, protocol amendments, and participant interactions. This practice is essential for compliance with regulations and data integrity, especially under the scrutiny of health authorities.
-
Training and Education: Ensure that all team members are adequately trained in the research protocol and compliance requirements. Ongoing education can improve team performance and adherence, ultimately aiding the effective navigation of intricate regulatory landscapes for medical device research.
By adhering to these best practices, researchers can boost the effectiveness of their feasibility assessments, contributing to the successful advancement of medical devices in Latin America, especially in markets like Colombia. With over 20 years of experience in Medtech, bioaccess® is well-equipped to manage Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), and other clinical trials, ensuring comprehensive support throughout the process.
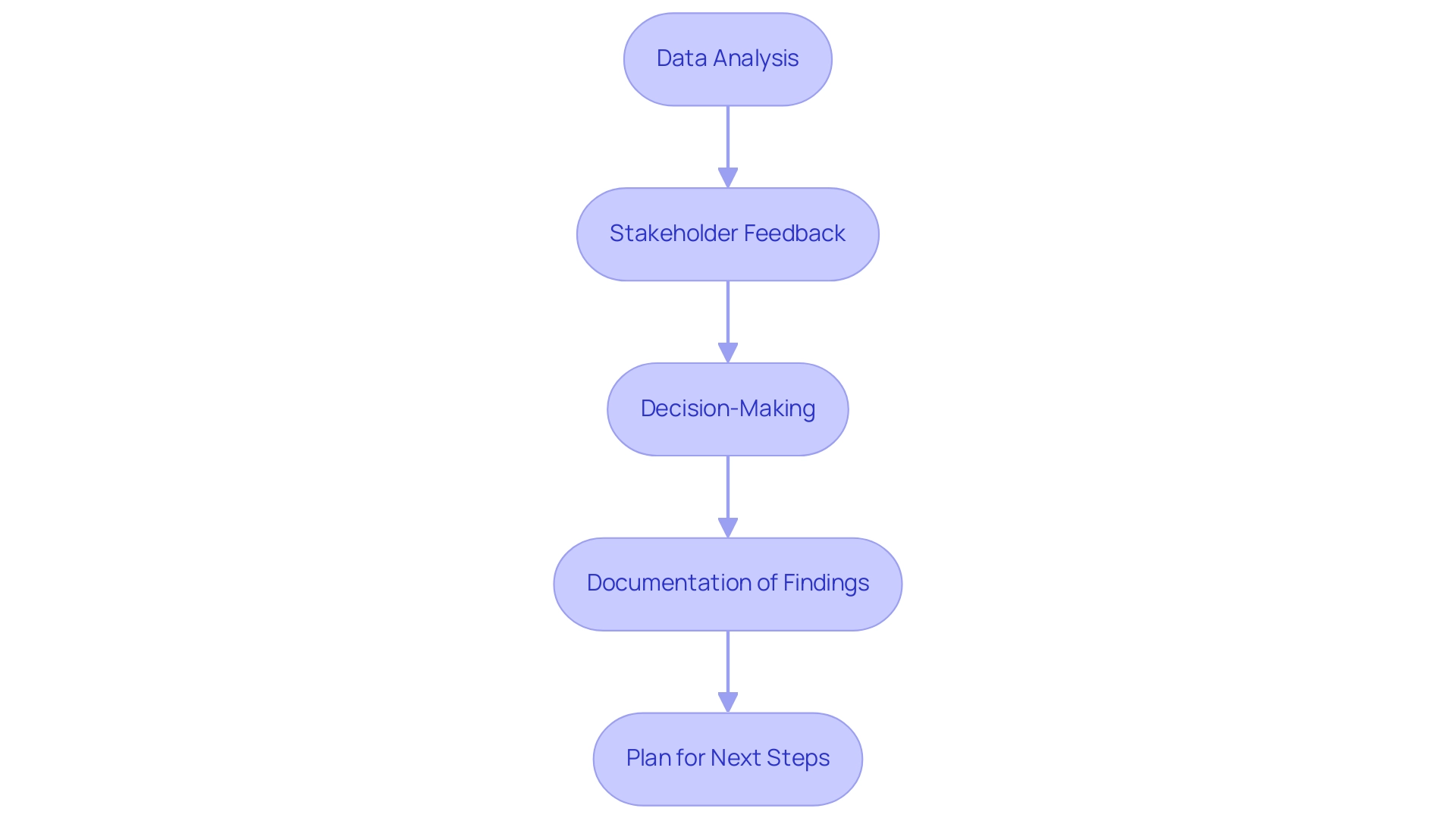
Evaluating the Results of Feasibility Studies
After completing the feasibility analysis, researchers should take the following steps to evaluate the results:
-
Data Analysis: Conduct a thorough analysis of the collected data, comparing it against the pre-defined objectives and hypotheses. Look for patterns, successes, and areas for improvement.
-
Stakeholder Feedback: Collect input from stakeholders involved in the research, including participants, clinicians, and regulatory representatives. Their insights can provide valuable context for interpreting the results.
-
Decision-Making: Use the findings to inform decisions about the next steps in the development process. This may include modifications to the apparatus design, further research, or proceeding to clinical trials, such as those seen in ReGelTec's Early Feasibility Study with HYDRAFIL™, where a patented hydrogel was successfully injected into the nucleus of degenerated discs, and Flow-FX's first-in-human trial with the Flow-Screw apparatus, designed for intraosseous antibiotic delivery, which demonstrated promising initial outcomes.
-
Documentation of Findings: Prepare a detailed report summarizing the results, methodologies, and recommendations. This report should be shared with all relevant stakeholders to maintain transparency and facilitate informed decision-making.
-
Plan for Next Steps: Based on the evaluation, develop a clear plan outlining the next stages of research or development. Ensure that this plan addresses any identified challenges and leverages the insights gained from the feasibility analysis. Given the complexities of navigating regulatory environments in Latin America, including challenges such as varying compliance requirements and the need for local expertise, consider utilizing comprehensive clinical trial management services, such as those offered by bioaccess®, which have proven instrumental in facilitating site selection, compliance reviews, trial setup, and project management in the successful execution of these studies.
By effectively evaluating the results of feasibility studies, researchers can make informed decisions that enhance the likelihood of successful medical device development in Chile.
Conclusion
Early feasibility studies are essential in the medical device development process, serving as a critical step to assess the viability of new innovations before entering full-scale clinical trials. These preliminary investigations not only gather vital safety and effectiveness data but also help navigate the complex regulatory landscapes, particularly in regions like Chile and Latin America. By identifying potential challenges early, developers can fine-tune their designs and methodologies, increasing the likelihood of successful outcomes.
The step-by-step process outlined for conducting feasibility studies in Chile underscores the importance of meticulous planning, regulatory compliance, and stakeholder engagement. Collaborations, such as those facilitated by bioaccess™, demonstrate how strategic partnerships can streamline the complexities of regulatory requirements, ultimately enhancing the efficiency of clinical trials. Adhering to best practices, including continuous monitoring and thorough documentation, further ensures that studies meet ethical standards and regulatory expectations.
In conclusion, embracing early feasibility studies is not merely a regulatory obligation; it is a strategic approach to fostering innovation in medical technology. By leveraging expert guidance and robust methodologies, researchers can navigate the intricacies of the regulatory environment, leading to advancements that significantly improve patient care. As the Medtech industry continues to evolve, the emphasis on these initial studies will be pivotal in driving successful medical device development and ensuring that innovations translate into real-world benefits for patients and healthcare systems alike.




