Introduction
In the dynamic landscape of medical device development, early feasibility studies play a pivotal role in determining the viability of innovative solutions before they enter larger clinical trials. In Colombia, the regulatory environment, primarily governed by INVIMA, presents both challenges and opportunities for researchers aiming to navigate this complex process. Recent advancements, such as eyeFlow, Inc.'s pilot study on a groundbreaking glaucoma treatment, underscore the importance of understanding local regulations and stakeholder engagement.
This article delves into the essential steps required for conducting effective feasibility studies, highlighting the significance of regulatory compliance, ethical considerations, and collaboration with key stakeholders. By exploring these critical elements, researchers can enhance their understanding and execution of early feasibility studies, ultimately contributing to the advancement of medical technology in the region.
Understanding Early Feasibility Studies in Colombia's Medical Device Landscape
Early feasibility evaluations are preliminary investigations conducted to assess the viability of a medical device before larger-scale clinical trials. In Colombia, grasping the regulatory structure established by the National Institute for Drug and Food Surveillance is essential, particularly after eyeFlow, Inc.'s recent authorization for an 18-month pilot trial on an innovative glaucoma treatment and PAVmed's successful first-in-human implantations of its PortIOn™ Intraosseous Infusion System. These investigations assist in identifying potential risks, refining protocols, and collecting initial data on safety and performance. Key aspects to consider include:
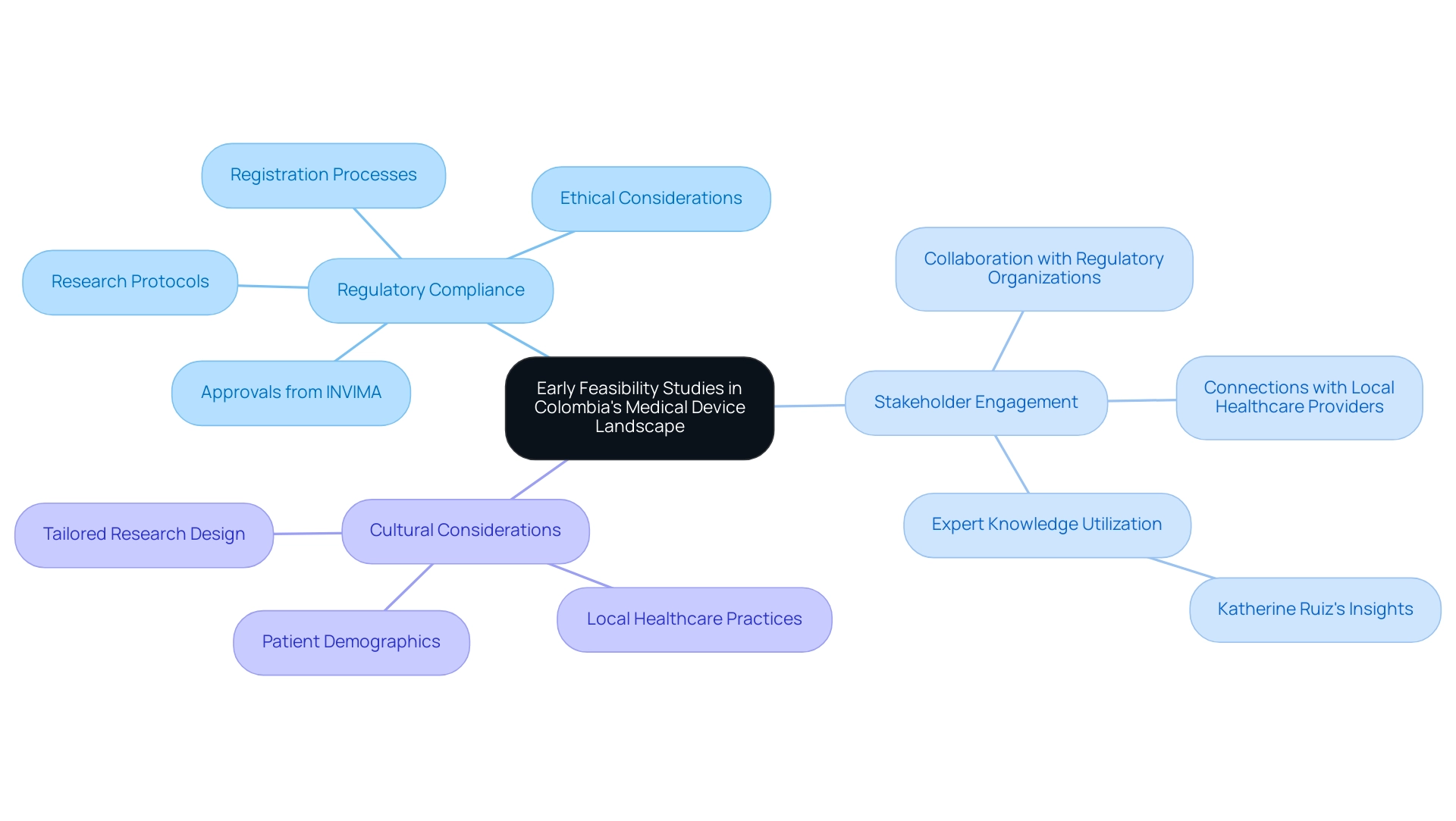
- Regulatory Compliance: Familiarize yourself with Colombian regulations governing healthcare products, including registration processes, ethical considerations, and specific compliance steps such as submitting research protocols for review and obtaining necessary approvals from INVIMA, a Level 4 health authority recognized by PAHO/WHO.
- Stakeholder Engagement: Build connections with local healthcare providers and regulatory organizations to enable more seamless project execution, utilizing knowledge from experts such as Katherine Ruiz, who focuses on regulatory matters for healthcare products in Colombia.
- Cultural Considerations: Understand the local healthcare practices and patient demographics to tailor your research design appropriately.
By grasping these elements, researchers can effectively navigate the complexities of conducting early feasibility assessments in Colombia, bridging the gaps in clinical research and innovation. Furthermore, it is essential to create plans for initial data gathering and to evaluate possible hazards linked to the use of new healthcare instruments during these assessments.
Step-by-Step Guide to Conducting Feasibility Studies for Medical Devices
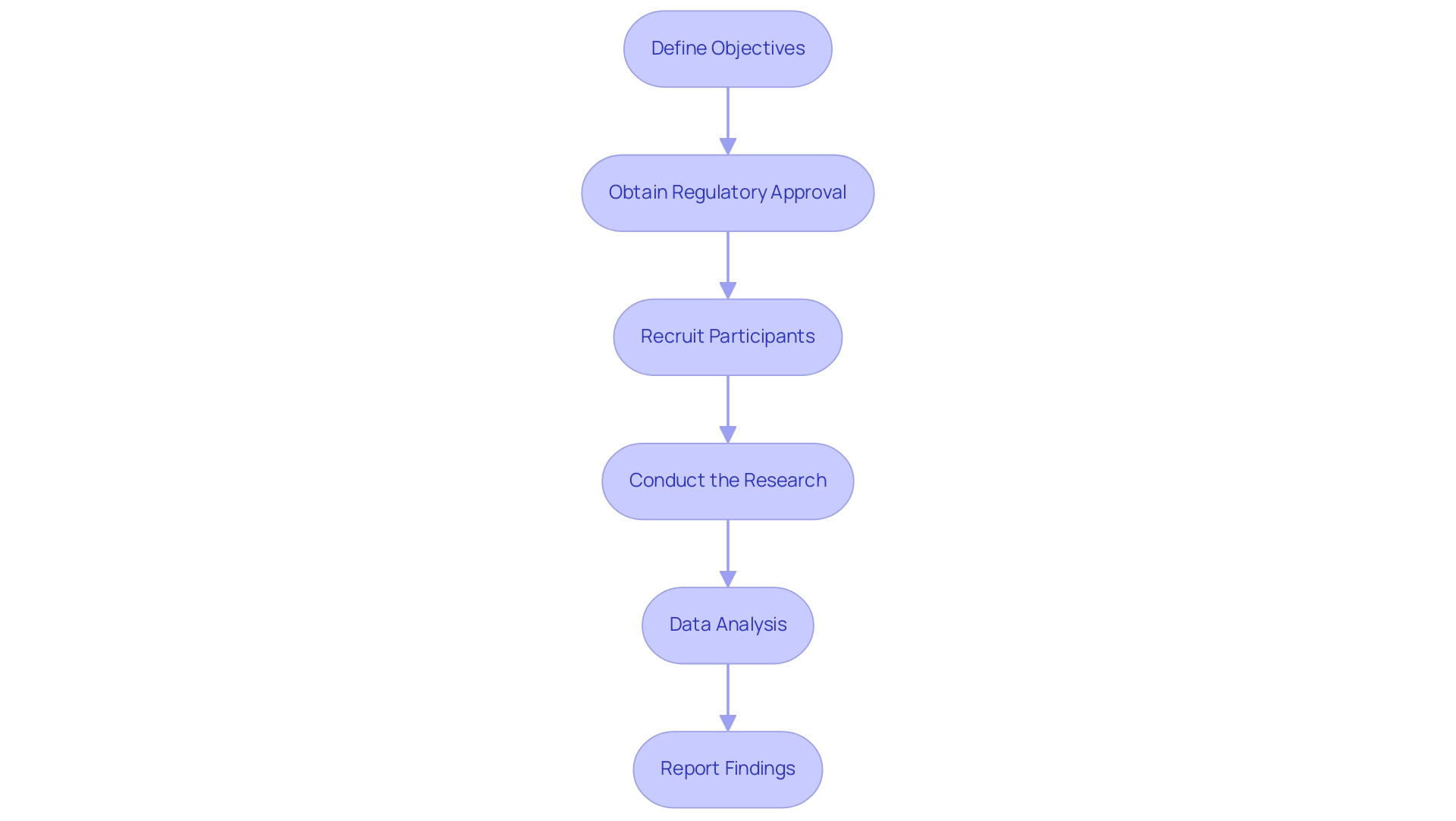
To carry out initial viability assessments for medical instruments in Colombia effectively, follow these structured steps:
- Define Objectives: Clearly outline the goals of your feasibility assessment, including specific questions regarding the device's safety and efficacy.
Develop a Research Protocol: Create a detailed protocol encompassing research design, participant selection criteria, and data collection methods. Ensure thorough compliance with local regulations, taking into account the specific requirements set forth by INVIMA. - Obtain Regulatory Approval: Submit your research protocol to the authority for review and approval. This step is crucial for legal compliance and ethical considerations, as INVIMA oversees the marketing and manufacturing of health products in Colombia.
- Recruit Participants: Identify and recruit a representative sample of participants, ensuring informed consent is obtained in accordance with ethical guidelines, as is standard practice in clinical trials.
- Conduct the Research: Execute the research according to the approved protocol, closely monitoring for any adverse events or unexpected outcomes. Bioaccess® offers comprehensive project management and monitoring services to assist in this phase, ensuring that all aspects of the trial are meticulously managed.
- Data Analysis: Examine the information gathered during the research to evaluate the feasibility of the apparatus, identifying any essential adjustments for upcoming trials. Bioaccess® provides expertise in data analysis to ensure accurate interpretation of results.
- Report Findings: Prepare a comprehensive report summarizing the research outcomes, including recommendations for further development or modifications to the equipment. This reporting is essential for compliance and to inform future research, and bioaccess® assists in ensuring that all reporting meets regulatory standards.
By meticulously following these steps and leveraging the expertise of professionals like Katherine Ruiz, researchers can ensure that their early feasibility evaluations are well-structured, compliant with Colombian regulations, and ultimately contribute to the advancement of medical devices in the region. Furthermore, case examples demonstrating the success of bioaccess®'s services in early-feasibility assessments can further illustrate the effectiveness of their approach.
Identifying Key Stakeholders and Building Collaborations
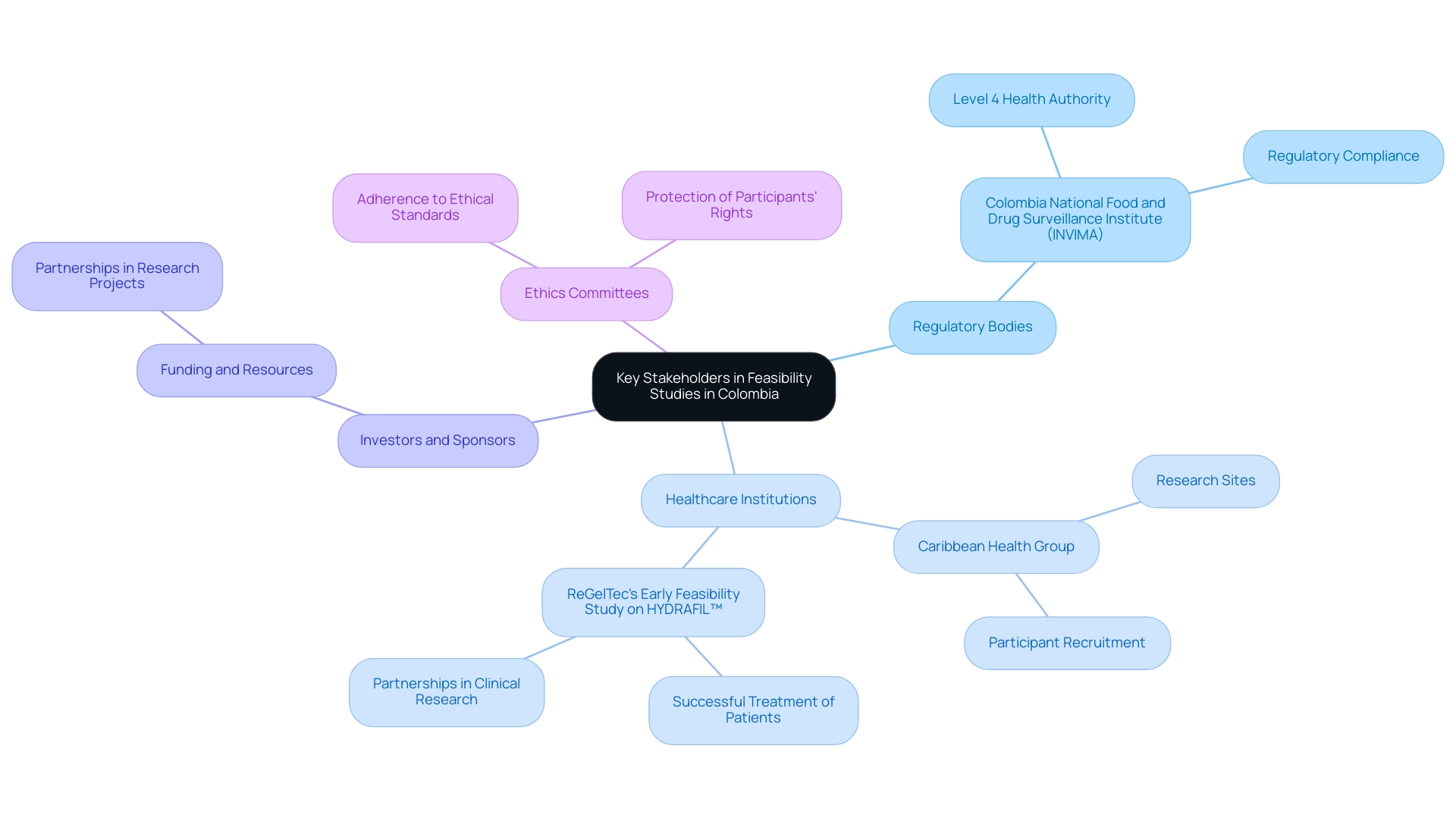
In Colombia, key stakeholders in early feasibility studies include:
- Regulatory Bodies: Engage with the Colombia National Food and Drug Surveillance Institute to ensure compliance with regulations and gain insights into the approval process. INVIMA's classification as a Level 4 health authority by PAHO/WHO underscores its importance in medical device oversight.
- Healthcare Institutions: Collaborate with hospitals and clinics, such as those affiliated with the Caribbean Health Group, that may act as research sites to recruit participants and gather essential data. This collaboration is exemplified by ReGelTec's Early Feasibility Study on HYDRAFIL™, which successfully treated eleven patients, demonstrating the effectiveness of partnerships in clinical research.
- Investors and Sponsors: Establish connections with potential investors or sponsors who can provide funding and resources for the research, similar to the partnerships formed in projects like ReGelTec's Early Feasibility Study on HYDRAFIL™.
- Ethics Committees: Work closely with ethics committees to ensure that the research adheres to ethical standards and that participants' rights are protected, fostering trust and integrity in research efforts.
Building strong relationships with these stakeholders can enhance the feasibility project's credibility and facilitate smoother execution. Regular communication and collaboration, as seen in the partnership between GlobalCare Clinical Trials and bioaccess™ to improve clinical trial services in Colombia, can lead to valuable insights and support throughout the research process, driving successful outcomes in clinical trials.
Navigating Regulatory Requirements and Ethical Considerations
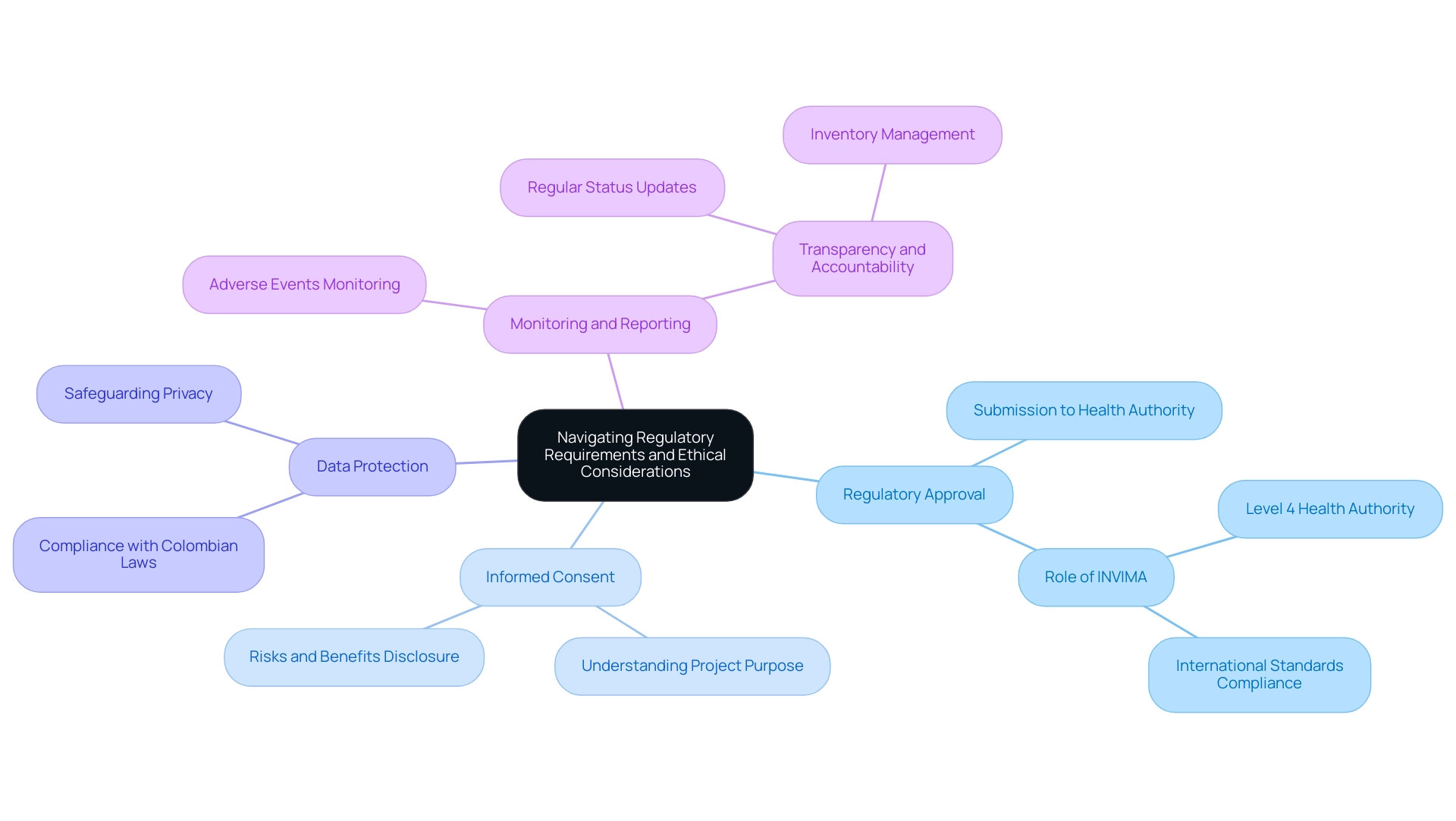
Conducting early feasibility assessments in Colombia requires adherence to both regulatory and ethical standards. Key considerations include:
- Regulatory Approval: Ensure that all research protocols are submitted to the health authority for approval prior to commencing the research. INVIMA operates as a Level 4 health authority, ensuring strict oversight and compliance with international standards.
- Informed Consent: Obtain informed consent from all participants, ensuring they understand the project's purpose, procedures, risks, and benefits.
- Data Protection: Implement measures to protect participants' personal data in compliance with Colombian data protection laws, safeguarding their privacy throughout the study.
- Monitoring and Reporting: Establish a system for monitoring adverse events and reporting them to regulatory bodies as required. This includes regular updates on research status and inventory management, ensuring transparency and accountability.
By navigating these regulatory requirements and ethical considerations, researchers can leverage Colombia's competitive advantages—such as cost savings of over 30% compared to North America, a swift regulatory review process of 90-120 days, high-quality healthcare ranked #22 by the World Health Organization, and robust patient recruitment facilitated by universal healthcare coverage—to protect participants and maintain the integrity of their investigations. Furthermore, our extensive clinical trial management services, including feasibility assessments, compliance reviews, and project oversight, guarantee that all facets of the trial are meticulously managed, further improving the efficiency and effectiveness of the research process.
Analyzing and Reporting Study Results
After conducting the feasibility assessment, the next steps involve analyzing and reporting the results:
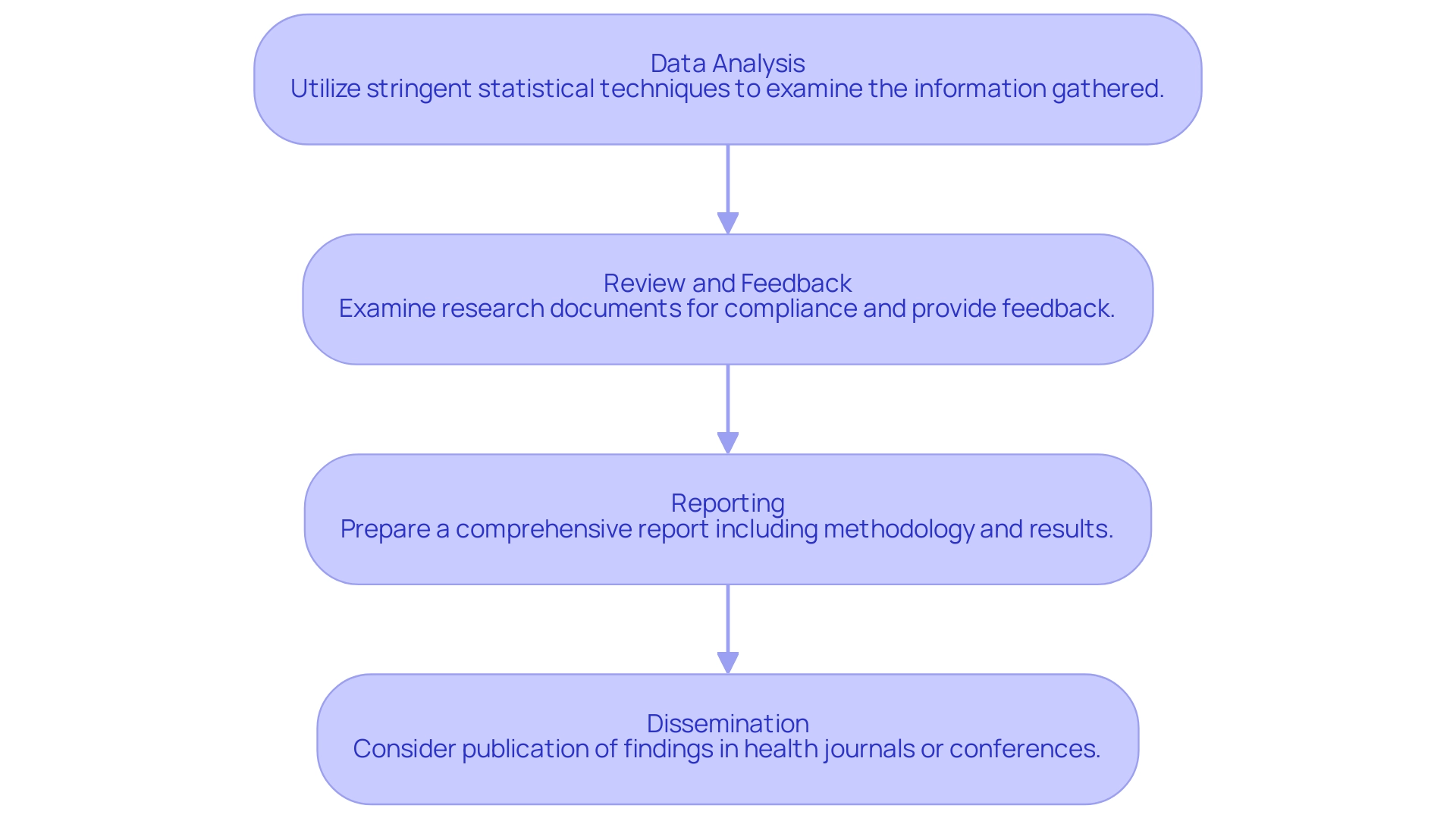
- Data Analysis: Utilize stringent statistical techniques to examine the information gathered during the research, concentrating on essential metrics that correspond with the research's goals and adhere to regulations.
Interpretation of Results: Contextualize the findings within the research objectives, addressing any limitations or unexpected outcomes that may arise. - Review and Feedback: Conduct a thorough examination of research documents to ensure compliance with country requirements, providing feedback to align with regulatory standards.
Trial Setup and Site Selection: Ensure proper trial setup and select appropriate research sites and principal investigators, facilitating effective project management throughout the study. - Reporting: Prepare a comprehensive report that includes an introduction, methodology, results, discussion, and conclusions, ensuring adherence to the regulatory requirements for submission.
Monitoring and Reporting Adverse Events: Implement a robust monitoring system to report serious and non-serious adverse events, ensuring participant safety and regulatory compliance. - Dissemination: Strategically consider the publication of findings in relevant health journals or presentations at conferences to share insights and advancements in healthcare technology with the broader healthcare community.
By meticulously following these steps, researchers can effectively analyze and report their study results. This systematic approach not only supports compliance with INVIMA but also contributes to the advancement of medical devices and the overall improvement of patient outcomes in Colombia, reflecting the successful collaborations exemplified by PAVmed's recent first-in-human implantations of the PortIO™ Intraosseous Infusion System.
Conclusion
Early feasibility studies are essential in the medical device development process, particularly within Colombia's unique regulatory environment governed by INVIMA. By understanding and adhering to local regulations, researchers can effectively navigate the complexities of conducting these studies. The significance of regulatory compliance, stakeholder engagement, and ethical considerations cannot be overstated, as they form the foundation for successful research outcomes.
The structured approach outlined in the article provides a clear pathway for researchers to follow, from defining study objectives to reporting findings. Each step is critical in ensuring that studies are conducted ethically and efficiently, ultimately contributing to the advancement of medical technology in the region. Collaborating with local healthcare institutions and regulatory bodies enhances the credibility of studies while fostering an environment conducive to innovation.
In summary, early feasibility studies not only identify potential risks and refine study protocols but also play a crucial role in bridging the gap between research and clinical application. By embracing the outlined strategies and focusing on regulatory adherence, researchers can significantly impact the medical device landscape in Colombia, paving the way for future advancements that improve patient care and outcomes. The commitment to thorough, ethical research will serve as a catalyst for innovation, ultimately benefiting both the scientific community and the patients they serve.




