Introduction
In the dynamic landscape of medical device development, early feasibility studies play a pivotal role, particularly in the diverse and complex environments of Latin America. These preliminary evaluations not only assess the feasibility and safety of innovative devices but also navigate the unique regulatory frameworks and healthcare practices that vary across the region. As manufacturers seek to bridge the gap between innovation and execution, understanding the significance of local insights becomes essential.
This article delves into the intricacies of conducting early feasibility studies in Latin America, exploring key processes, stakeholder engagement, and the critical steps needed to ensure successful outcomes in this evolving market.
Understanding Early Feasibility Studies in Latin America
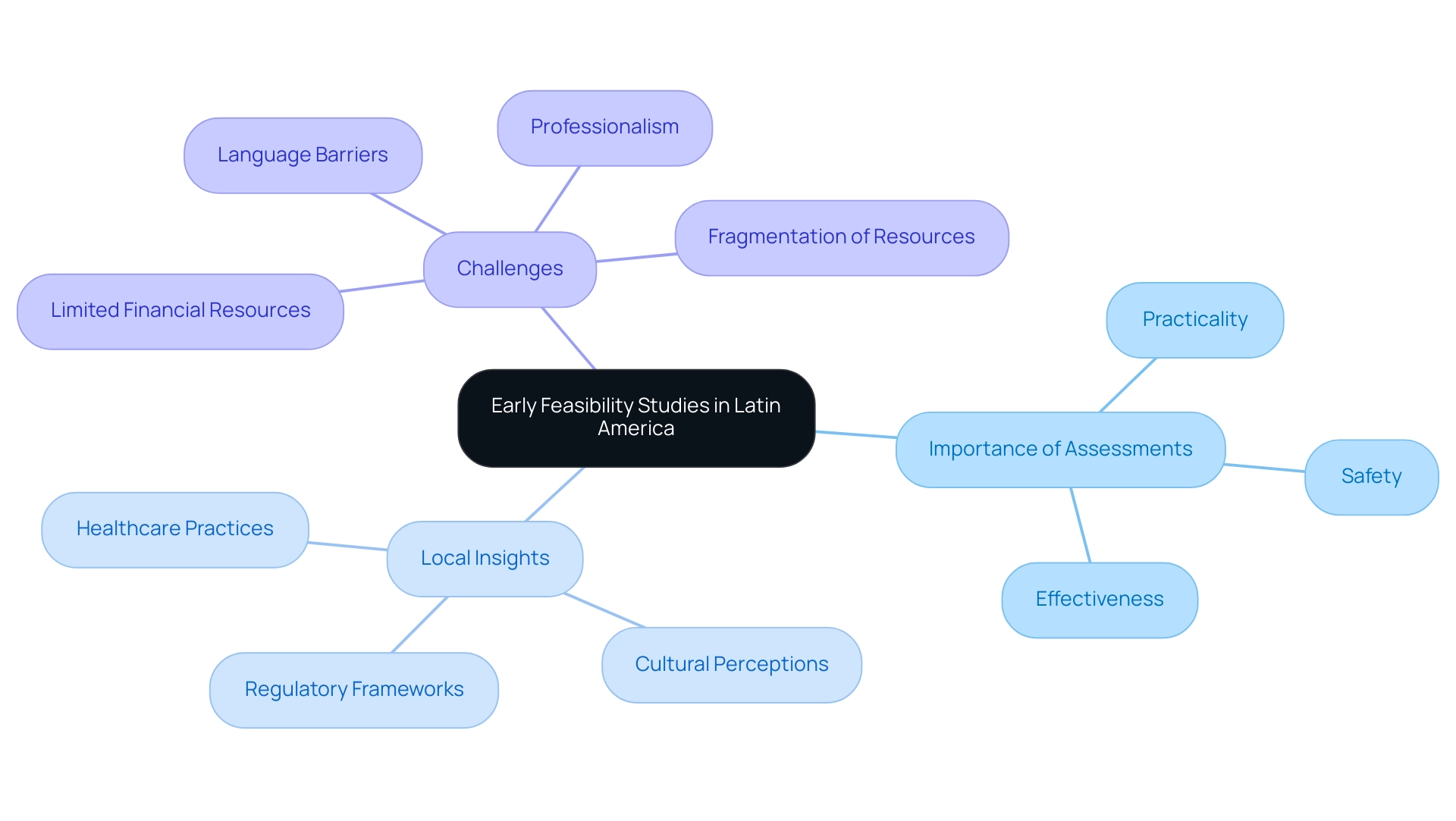
Initial assessments play a vital introductory stage in the advancement of medical equipment, especially in Latin America. These investigations are intended to assess the practicality, safety, and possible effectiveness of an apparatus prior to progressing to larger clinical trials. They involve smaller groups of participants, allowing researchers to gather initial data on device performance and user experience. In Latin America, the significance of the research efforts is heightened due to varying regulatory frameworks, local healthcare practices, and market needs. Conducting early feasibility assessments helps manufacturers identify potential hurdles related to local regulations, cultural perceptions, and healthcare practices. Notable examples include:
- PAVmed's first-in-human study in Colombia
- ReGelTec's successful treatment of chronic low back pain patients with HYDRAFIL™
Both showcasing how local insights can enhance the effectiveness of their products. Additionally, Avantec Vascular's partnership with bioaccess™ for its innovative vascular technology and Flow-FX's first-in-human clinical trial on the Flow-Screw instrument underscore the power of collaboration in overcoming regional challenges.
However, US Medtech companies also face broader challenges in Latin America, including:
- Limited financial resources
- Professionalism
- Language barriers
- Fragmentation of resources
These factors can impede effective communication and collaboration with local hospitals. By addressing these factors early on, researchers can enhance their instruments and strategies, ultimately leading to more successful outcomes when entering the market. Moreover, this research fosters collaboration with local stakeholders, enhancing the understanding of regional healthcare challenges and patient needs, and bridging the gap between innovation and execution.
Navigating the Medical Device Registration Process in Panama
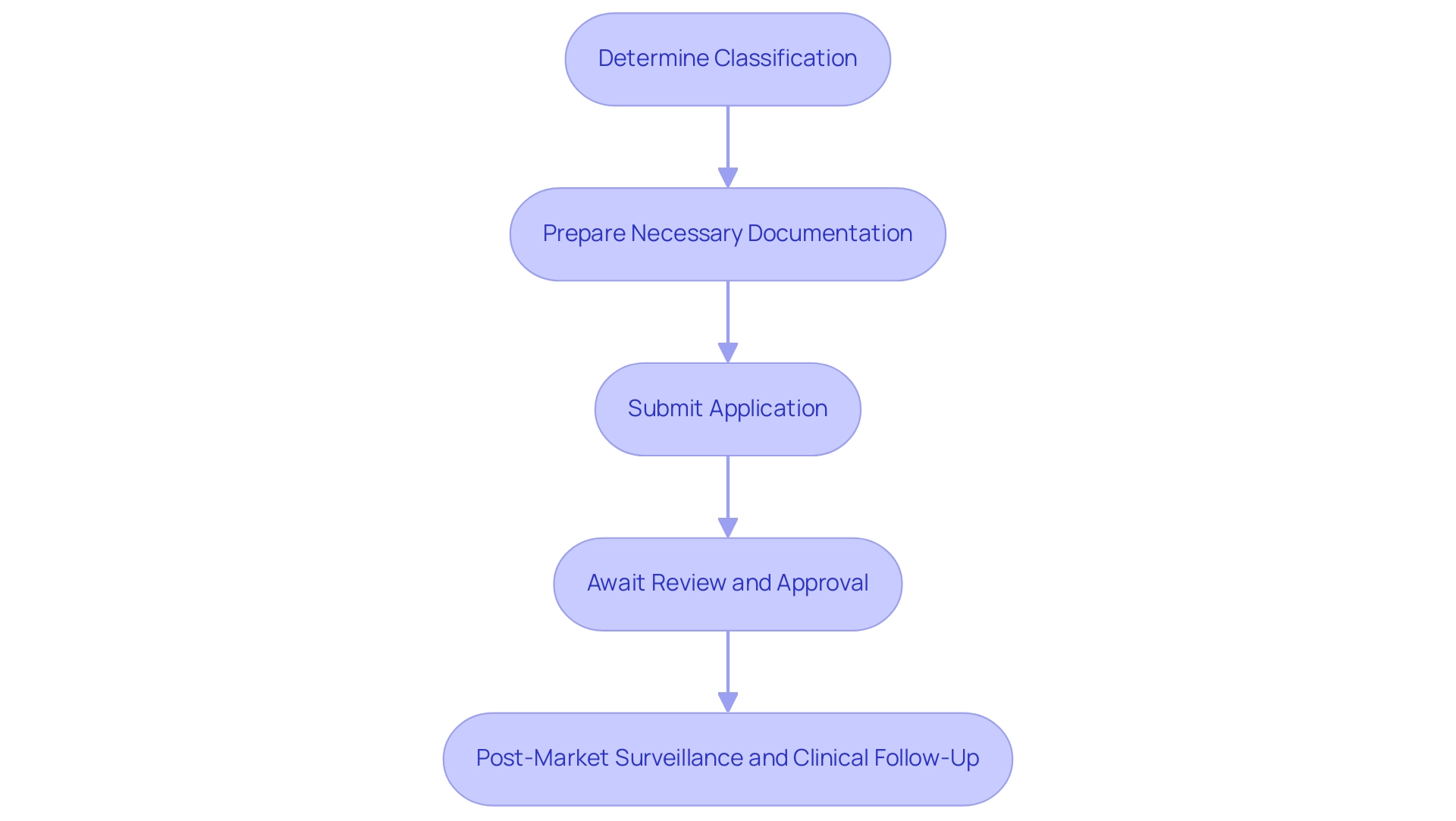
To successfully conduct early feasibility studies for medical equipment in Panama, it is essential to navigate the local medical equipment registration process, which requires a thorough understanding of regulatory frameworks. This process typically involves several key steps:
-
Determine Classification: Medical instruments in Panama are categorized based on risk levels. Comprehending your gadget's classification is essential, as it determines the regulatory requirements for registration.
-
Prepare Necessary Documentation: Gather all required documentation, including technical specifications, clinical data, and a summary of the intended use. This documentation must comply with the regulations set forth by the Panamanian Ministry of Health, reflecting the expertise of bioaccess® in managing compliance reviews.
-
Submit Application: Applications for medical equipment registration must be submitted to the Ministry of Health. Ensure that all documents are complete and accurately reflect the specifications of the equipment to avoid delays, leveraging the comprehensive support provided by bioaccess® in trial setup, project management, and monitoring.
-
Await Review and Approval: The review process can take several weeks to months, depending on the complexity of the apparatus and the completeness of the application. Be ready to address any inquiries for further details from regulatory authorities, utilizing the expertise of Katherine Ruiz, a specialist in Regulatory Affairs for medical products in Colombia.
-
Post-Market Surveillance and Clinical Follow-Up: Once approved, ongoing monitoring of the product’s performance in the market is essential. This includes reporting any adverse events or issues that may arise, ensuring continued compliance with regulatory standards. The significance of post-market clinical follow-up research (PMCF) cannot be exaggerated, as they play a crucial role in assessing the long-term safety and effectiveness of the equipment.
By following these steps and complying with the medical equipment registration process in Panama, researchers can effectively carry out early viability assessments, paving the way for successful product development and market entry with the support of bioaccess®'s accelerated clinical research services, backed by over 20 years of experience in the Medtech industry.
Identifying and Engaging Key Stakeholders
Recognizing and involving essential stakeholders is a crucial phase in performing initial assessments. Stakeholders may include:
- Healthcare Professionals: Engaging physicians, nurses, and other healthcare providers can provide valuable insights into clinical practices and patient needs, addressing the challenges of recruiting participants for clinical trials.
- Regulatory Authorities: Establishing communication with local regulatory bodies, such as INVIMA in Colombia, is essential. Comprehending their regulatory roles and requirements can ease a smoother registration process and ensure adherence to regulations, thereby navigating potential obstacles effectively.
- Patients and Patient Advocacy Groups: Engaging patients in the design of the research not only assists researchers in grasping patient viewpoints but also improves recruitment strategies, resulting in more pertinent and effective solutions.
- Investors and Sponsors: Establishing connections with potential investors or sponsors is crucial for obtaining the necessary funding and resources for conducting viability assessments, addressing the financial challenges many medical equipment startups encounter.
To effectively involve these stakeholders, consider the following strategies:
- Conduct Informational Meetings: Host meetings or webinars to inform stakeholders about the objectives of the research and gather feedback, promoting collaboration and support.
- Create Collaborative Platforms: Utilize online collaboration tools to facilitate ongoing communication and input from stakeholders throughout the research, ensuring their expertise and insights are continuously integrated.
- Involve Stakeholders in Research Design: Encourage stakeholders to participate in the development of research protocols to ensure their insights are included, thus enhancing the quality and relevance of the assessments.
By cultivating strong relationships with key stakeholders, researchers can improve the quality and relevance of their early viability assessments, ultimately leading to better outcomes and increased acceptance of new medical equipment. Furthermore, these engagements can facilitate compliance with regulatory requirements and positively impact local economies through job creation and healthcare improvement, demonstrating the broader benefits of successful clinical trials.

Designing the Early Feasibility Study Protocol
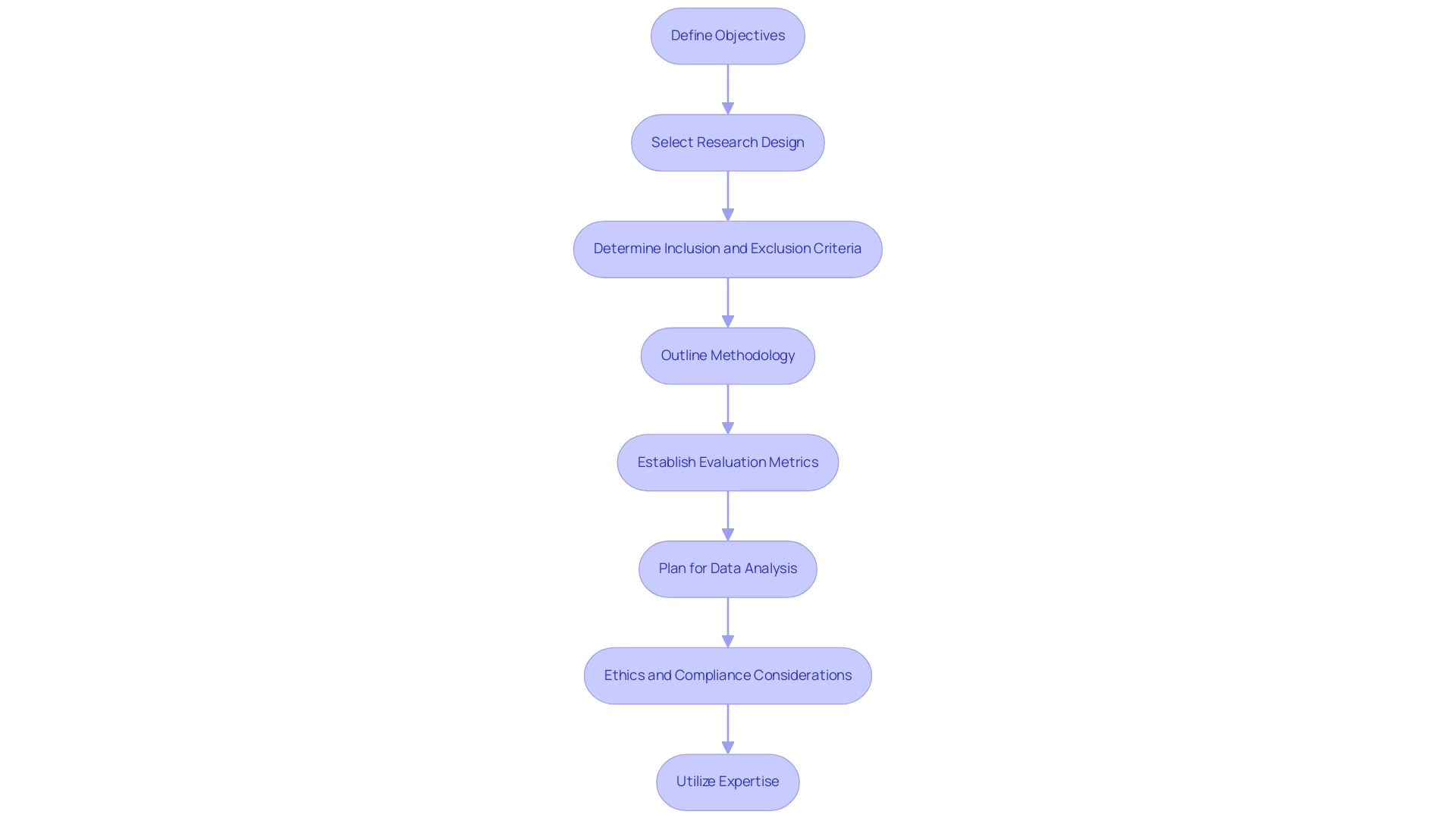
Creating an effective initial assessment protocol involves several key components, especially when navigating the regulatory environments in Latin America:
- Define Objectives: Clearly outline the primary objectives of the research, including specific questions the investigation aims to answer regarding the device’s feasibility and performance.
- Select Research Design: Choose an appropriate research design that aligns with the objectives. Common designs include single-arm trials, pilot projects, and observational research, all of which bioaccess® can assist with through their extensive experience.
- Determine Inclusion and Exclusion Criteria: Establish criteria for participant selection to ensure that the population is representative and relevant to the intended use of the device.
- Outline Methodology: Describe the research procedures, including how the equipment will be utilized, information collection techniques, and any evaluations that will be performed to assess outcomes.
- Establish Evaluation Metrics: Define clear metrics for assessing the performance of the apparatus, including safety, usability, and initial efficacy indicators.
- Plan for Data Analysis: Describe the statistical methods that will be used to analyze the collected data, ensuring that they are appropriate for the research design and objectives.
- Ethics and Compliance Considerations: Ensure that the protocol adheres to ethical standards and regulatory requirements, including obtaining informed consent from participants. Bioaccess® offers vital support in reviewing and providing feedback on research documents to comply with country requirements, facilitating trial setup, and ensuring import permits for investigational devices.
- Utilize Expertise: With over 20 years of experience in Medtech, bioaccess® offers customized assistance in site selection, project management, and detailed reporting on trial status and adverse events, ensuring a thorough approach to clinical trial management.
By carefully designing the research protocol and utilizing the comprehensive clinical trial management services available, including site selection and project management, researchers can create a solid framework for conducting early feasibility assessments. This increases the likelihood of obtaining meaningful results that inform future development stages.
Implementing the Study and Collecting Data
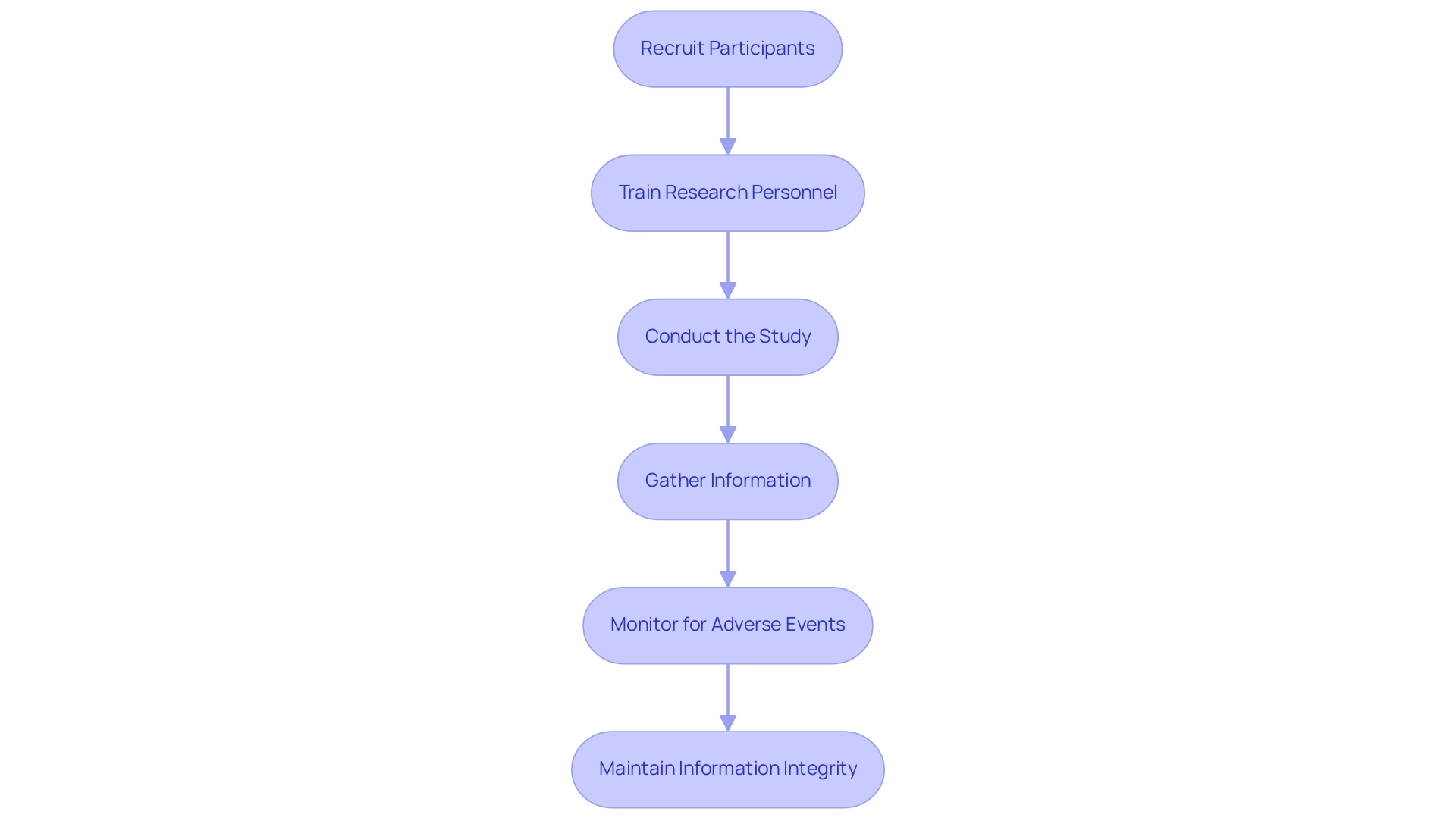
Once the research protocol is developed, the next step is to execute the research and gather information. Here are key steps to ensure effective execution:
-
Recruit Participants: Utilize the defined inclusion and exclusion criteria to recruit suitable participants. Ensure that recruitment processes are ethical and transparent.
-
Train Research Personnel: Provide comprehensive training for all research personnel to ensure they understand the protocol, device usage, and information collection methods. Proper training is essential for maintaining consistency and reliability in information collection.
-
Conduct the Study: Follow the protocol closely during the study execution. Monitor the participants and ensure that the device is used as intended, gathering information at predetermined intervals.
-
Gather Information: Use standardized forms and tools to collect information systematically. Ensure that information collection methods are consistent and that all relevant details are recorded accurately.
-
Monitor for Adverse Events: Continuously observe participants for any adverse events or complications associated with the equipment. Establish a reporting mechanism to document and address any issues promptly.
-
Maintain Information Integrity: Implement quality control measures to ensure the integrity of the collected information. Consistently assess information for thoroughness and precision.
By efficiently executing the research and gathering high-quality data, investigators can extract valuable insights from initial feasibility assessments, ultimately guiding subsequent phases of medical equipment development. With bioaccess®'s extensive expertise in managing early-feasibility, first-in-human, and pivotal studies, along with over 20 years of experience in Medtech, you can trust that every aspect of your clinical trial is supported by seasoned professionals committed to compliance and excellence in Latin America. Additionally, as the only vetted CRO and consulting partner for U.S. medical device companies in Colombia, bioaccess® provides critical services such as site selection and compliance reviews to ensure successful outcomes.
Conclusion
Early feasibility studies are integral to the successful development of medical devices in Latin America, providing a framework for assessing both the feasibility and safety of innovative solutions. These studies are particularly crucial in a region characterized by diverse regulatory requirements and healthcare practices. By engaging local stakeholders, including healthcare professionals and regulatory authorities, manufacturers can navigate the complexities of the market, ensuring that their devices align with regional needs and expectations.
The process of conducting early feasibility studies involves several essential steps, from understanding the local medical device registration process to designing effective study protocols. Each phase requires careful consideration of objectives, participant selection, and data collection methods to yield meaningful insights. Furthermore, the emphasis on collaboration with local partners, such as bioaccess®, enhances the likelihood of successful outcomes by leveraging their expertise in regulatory compliance and clinical management.
In summary, the significance of early feasibility studies in Latin America cannot be overstated. By prioritizing local insights and stakeholder engagement, manufacturers can bridge the gap between innovation and execution, ultimately leading to the successful introduction of medical devices that address the specific needs of the region. As the medical landscape continues to evolve, embracing these studies will be vital for driving advancements in healthcare and improving patient outcomes across Latin America.




