Introduction
The introduction of innovative medical devices in the Dominican Republic hinges on the successful execution of early feasibility studies, a critical preliminary step for manufacturers. These studies not only assess the practicality and potential clinical benefits of new technologies but also provide invaluable insights into the local healthcare landscape. Understanding patient demographics and prevalent health issues is essential for tailoring research effectively, thereby increasing the likelihood of regulatory approval and market entry.
As the demand for advanced medical solutions grows, navigating the complexities of regulatory and ethical approvals becomes paramount. This article delves into the essential components of conducting early feasibility studies, from designing study protocols and recruiting participants to managing data collection, all while highlighting the role of expert services in facilitating these processes.
Understanding Early Feasibility Studies in the Dominican Republic
Initial feasibility assessments are preliminary inquiries aimed at determining the practicality and possible clinical advantages of a medical instrument. In the Dominican Republic, these examinations serve as a crucial step for manufacturers looking to introduce innovative medical technologies. Conducted as part of a comprehensive clinical trial management service, they typically involve small sample sizes and focus on gathering initial data about the product's functionality, safety, and potential effectiveness in a real-world setting.
Comprehending the local healthcare landscape—including patient demographics and common health concerns—is essential for customizing these analyses effectively. Such insights not only guide device development but also enhance the likelihood of successful regulatory approval and market entry, ultimately contributing to job creation and economic growth in the region.
bioaccess® provides a variety of services, including:
- Feasibility assessments
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Reporting
This ensures that all aspects of the clinical trial process are managed efficiently. However, Medtech companies in Latin America also face challenges such as regulatory hurdles and communication barriers, which can impede progress. The partnership between Greenlight Guru and bioaccess™ further exemplifies the commitment to accelerating Medtech innovations in Latin America, as highlighted by recent successes such as PAVmed's first-in-human trial in Colombia.
Navigating Regulatory and Ethical Approval for Medical Device Studies
To carry out early feasibility assessments for medical devices in the Dominican Republic, researchers must first obtain regulatory approval from the Ministry of Public Health. This involves submitting a comprehensive research protocol that outlines the project's objectives, methodology, and potential risks to participants. Additionally, ethical approval from an Institutional Review Board (IRB) is necessary to ensure that the rights and welfare of participants are safeguarded throughout the research.
Researchers should prepare to provide documentation such as:
- Informed consent forms
- Safety monitoring plans
- Data protection measures
Engaging with local regulatory experts and legal advisors can streamline this process, helping to address any specific local requirements or concerns that may arise.
bioaccess® provides extensive clinical study management services, including:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Study setup
- Import permits
- Nationalization of investigational tools
- Project management
- Reporting
Adhering to these regulations not only protects participants but also boosts the credibility of the research outcomes, paving a smoother way for medical startups confronting obstacles like regulatory challenges, competition from established firms, and issues in patient recruitment. With over 20 years of experience in Medtech, bioaccess® is well-equipped to navigate these complexities and support your medical equipment trials.
Designing the Study Protocol for Feasibility Studies
When creating a study protocol for an early feasibility investigation, researchers should incorporate the following key elements:
- A clear statement of the study objectives
- A detailed description of the medical apparatus being tested
- The study design (e.g., observational, interventional)
- The target population
Additionally, outline the inclusion and exclusion criteria for participant selection, specifying how bioaccess® can provide templates and guidance to ensure compliance with regulatory standards.
Detail the methodologies for information collection and analysis, where bioaccess® offers expertise in developing robust collection plans that align with regulatory requirements. It is also important to define the endpoints that will be measured to evaluate the performance and safety of the system.
By leveraging bioaccess®'s expertise in feasibility assessments and site selection, along with their comprehensive support in compliance evaluations and setup—including their experience with First-In-Human Experiments and Pilot Programs—researchers can facilitate smoother approvals and ensure robust data collection during the feasibility phase.
Recruiting Participants for the Study
Effective participant recruitment involves identifying the right channels to reach potential subjects. Researchers should leverage partnerships with local hospitals, clinics, and community organizations to raise awareness about the research, similar to how Welwaze Medical Inc. collaborated with bioaccess™ for the Celbrea® medical device launch in Colombia. Bioaccess™ offered crucial regulatory and market access consulting services, such as:
- Feasibility assessments
- Compliance reviews
- Setup for testing
These services aided Welwaze's entry into the Colombian market. Clear communication of the project's purpose, potential benefits, and risks is essential to build trust and encourage participation. Additionally, utilizing social media platforms and local health fairs can enhance visibility, as seen with GlobalCare Clinical Trials' efforts to expand clinical trial ambulatory services in the region, achieving over a 50% reduction in recruitment time and a 95% retention rate.
It is also crucial to ensure that recruitment materials are culturally sensitive and available in the primary language of the target population. By employing a thoughtful and ethical approach to recruitment, which includes respecting local customs and values, researchers can enhance participant engagement and retention throughout the research, ultimately contributing to job creation and improved healthcare in the local economy.
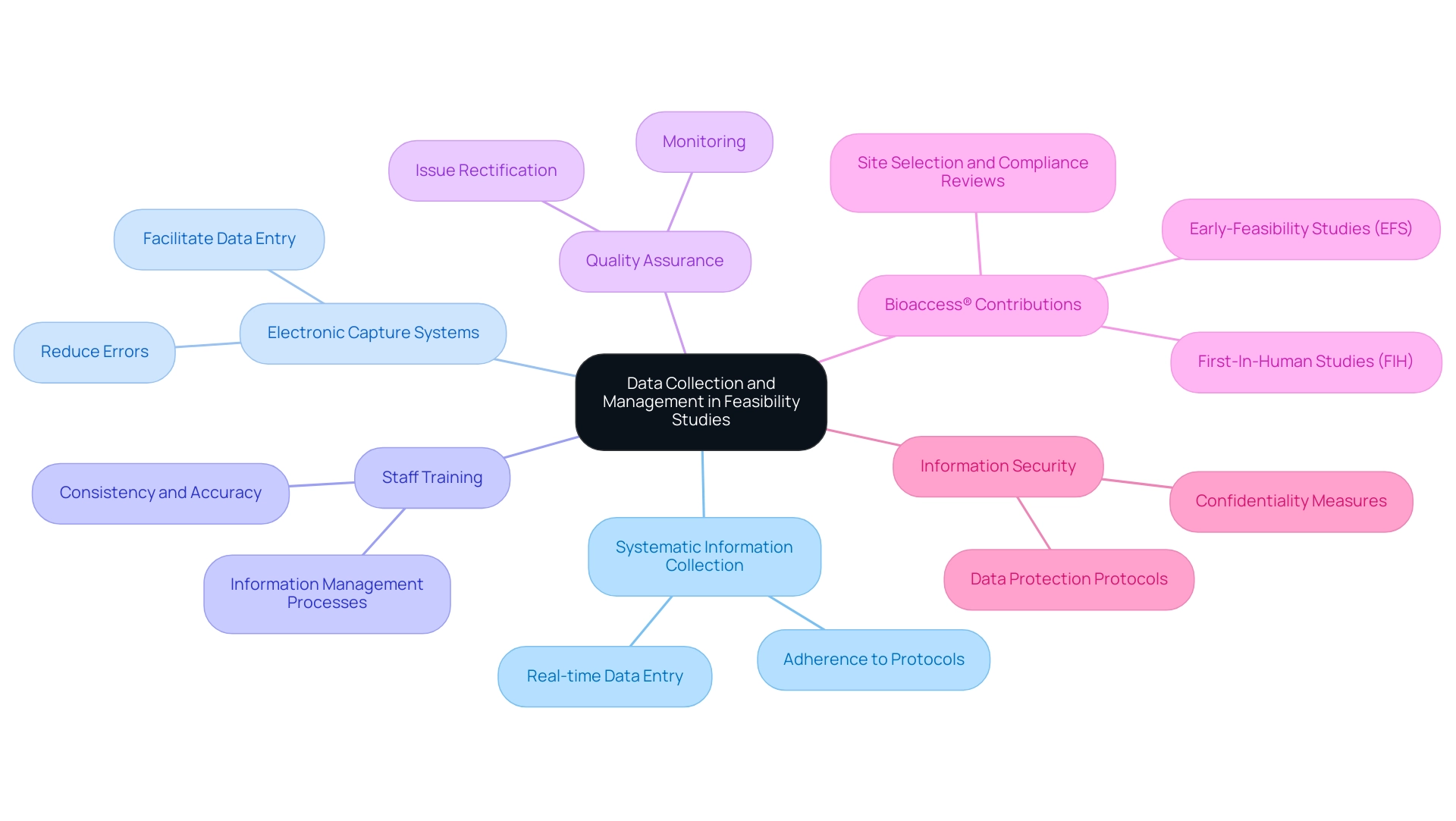
Data Collection and Management in Feasibility Studies
In feasibility studies, information collection should be systematic and adhere to the protocols established in the study design. Researchers should utilize electronic information capture systems to facilitate real-time entry and reduce errors. It is essential to train staff on information management processes to ensure consistency and accuracy in collection.
Additionally, leveraging the comprehensive clinical trial management services offered by bioaccess®, including site selection and compliance reviews, can significantly enhance the efficiency of these projects. bioaccess® specializes in Early-Feasibility Studies (EFS) and First-In-Human Studies (FIH), which require a flexible and specialized approach to navigate regulatory environments effectively.
Implementing information monitoring and quality assurance measures throughout the study can help identify and rectify issues early on. Researchers must also consider information security and participant confidentiality, ensuring that all information is stored securely and access is limited to authorized personnel.
bioaccess® tackles these challenges by establishing strong information protection protocols and ensuring adherence to local regulations. By prioritizing data integrity and utilizing expert services, researchers can enhance the credibility of their findings and support informed decision-making in subsequent phases of research.
Conclusion
The successful introduction of innovative medical devices in the Dominican Republic is intricately tied to the effective execution of early feasibility studies. These studies serve as a foundational step, allowing manufacturers to assess not only the practicality and potential clinical benefits of new technologies but also to gain a comprehensive understanding of the local healthcare landscape. By focusing on patient demographics and prevalent health issues, manufacturers can tailor their research to meet the specific needs of the population, thereby increasing the chances of regulatory approval and market entry.
Navigating the regulatory and ethical landscape is crucial for researchers conducting these studies. Obtaining the necessary approvals from the Ministry of Public Health and Institutional Review Boards ensures that participant rights and welfare are prioritized. By collaborating with local experts and leveraging comprehensive clinical trial management services, such as those provided by bioaccess®, researchers can overcome challenges associated with regulatory hurdles and improve the credibility of their findings.
Furthermore, the design of the study protocol and effective participant recruitment are vital components that contribute to the overall success of feasibility studies. A well-structured protocol, combined with strategic recruitment efforts that respect local customs and values, enhances participant engagement and retention. By utilizing electronic data capture systems and ensuring rigorous data management practices, researchers can maintain data integrity and support informed decision-making in subsequent research phases.
In summary, early feasibility studies are essential for the advancement of medical technologies in the Dominican Republic. By emphasizing thorough preparation, regulatory compliance, and effective data management, researchers and manufacturers can facilitate smoother pathways for innovation, ultimately benefiting the healthcare system and contributing to economic growth in the region.




